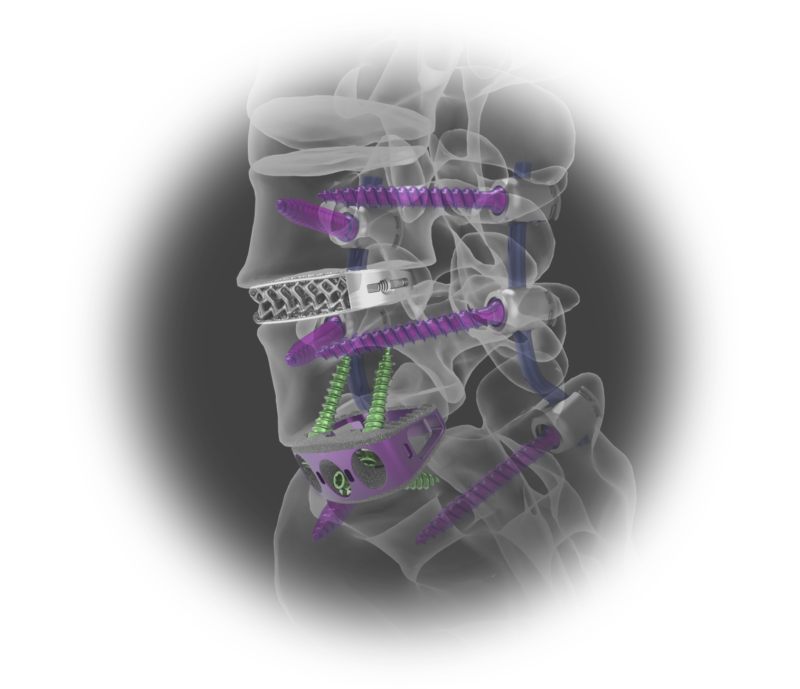
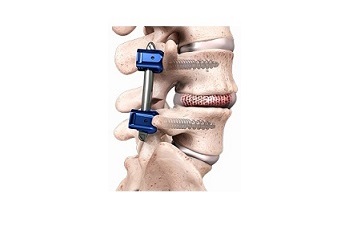
RAYNHAM, Mass., April 26, 2018 /PRNewswire/ — DePuy Synthes*, part of the Johnson & Johnson Medical Devices Companies**, today announces the U.S. launch of the PROTI 360° Integrated Titanium Family of interbody devices to advance care for patients who have degenerative disc disease in the neck and back. This new offering underscores the commitment of DePuy Synthes to expand the company’s portfolio of interbody fusion devices designed to help improve outcomes after spinal fusion surgery.
Approximately 400,000 patients undergo spinal fusion surgery annually to help reduce pain and nerve root inflammation in the US alone.1 During this procedure, a disc is removed and an interbody spacer is implanted to potentially restore natural height between two vertebrae and create solid bone.
To maximize the potential for bone growth following this type of surgery, DePuy Synthes has introduced the PROTI 360° Integrated Titanium Family of Implants, the only titanium-integrated Polyetheretherketone (PEEK) that offers 360 degrees of titanium integration. PEEK is a biocompatible material with mechanical properties that resemble human bone, and the implants are radiolucent, which means surgeons can easily assess the bone healing process over time.2The new PROTI 360° Technology offering combines the benefits of PEEK and titanium in each device. The titanium integrated surface of the PROTI 360° Family of Implants creates a bioactive surface that promotes the attachment and growth of bone-forming cells.3,4
Data presented at a recent podium presentation during the International Society for the Advancement of Spine Surgery Meeting showed calcium deposition, which is an indicator of early bone formation, was 470.4% higher with the PROTI 360° Technology than PEEK alone and 305.1% higher than titanium alone just one day after device testing.5 The PROTI 360° Family of Implants is designed with enhanced bond strength between the materials, fortified titanium edges, and no exposed corners to prevent delamination, a process in which titanium coatings separate from the PEEK implant where the two materials meet.
“The ability to maximize the potential for bone growth is fundamental to achieving the best possible patient outcomes in most reconstructive spinal fusion surgery,” said L. Erik Westerlund, MD, Surgeon and Director of the St. Francis Spine Center, Columbus, Georgia***. “Utilizing innovative biomaterials like titanium-integrated PEEK designed to promote bone growth delivers a comprehensive alternative offering to surgeons that advances the standard for spine surgery patients.”
The PROTI 360° Family of Implants includes the ACIS PROTI 360° System for cervical fusion and the T-PAL PROTI 360° System and CONCORDE PROTI 360° System for lumbar interbody fusion. The PROTI 360° Systems are compatible with ACIS, T-PAL and CONCORDE Systems instrument sets.
“With the launch of the PROTI 360° Family of Implants we are responding to the needs of spine surgeons with a differentiated technology to help advance the care of patients undergoing spinal fusion surgery,” said Nadav Tomer, Global Platform Leader, DePuy Synthes Spine. “DePuy Synthes has one of the broadest spine portfolios in the world, and we continue to focus on innovation that strengthens our offering.”
About the Johnson & Johnson Medical Devices Companies
The Johnson & Johnson Medical Devices Companies’ purpose is to reach more patients and restore more lives. Having advanced patient care for more than a century, these companies represent an unparalleled breadth of products, services, programs and research and development capabilities in surgical technology, orthopaedics, cardiovascular and specialty solutions with an offering directed at delivering clinical and economic value to health care systems worldwide.
About DePuy Synthes
DePuy Synthes, part of the Johnson & Johnson Medical Devices Companies, provides one of the most comprehensive orthopaedics portfolios in the world. DePuy Synthes solutions, in specialties including joint reconstruction, trauma, craniomaxillofacial, spinal surgery and sports medicine, are designed to advance patient care while delivering clinical and economic value to health care systems worldwide. For more information, visit www.depuysynthes.com.
*DePuy Synthes represents the products and services of DePuy Synthes, Inc. and its affiliates.
**The Johnson & Johnson Medical Devices Companies comprise the surgery, orthopaedics, and cardiovascular businesses within Johnson & Johnson’s Medical Devices segment.
***Consultant to DePuy Synthes Spine
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the PROTI 360° Integrated Titanium Family of interbody devices. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of DePuy Synthes, any of the other Johnson & Johnson Medical Devices Companies and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of health care products and services; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 31, 2017, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in the company’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither DePuy Synthes, the Johnson & Johnson Medical Devices Companies, nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
1Rajaee, Ss et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012 Jan 1;37(1):67-76.
2Nemoto, O. et al. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation. Eur Spine J. 2014 Oct;23(10):2150-5.
3Lincks, J. et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials 19, 1998. Pages 2219–32
4Kurtz, S. M. & Devine, J. N., PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007
5TR-201801-B (ProTi Test Report)
089999-180411
SOURCE DePuy Synthes