February 09, 2018
SUNNYVALE, Calif.–(BUSINESS WIRE)–Simplify Medical Pty Ltd, maker of the Simplify® cervical artificial disc, today announced a second tranche of its Series B financing of $23.25 million, completing the oversubscribed round totaling $44.25 million.
The lead investor for the second tranche is LSP Health Economics Fund 2 (LSP HEF 2), with participation from existing venture investors LSP Fund V, MH Carnegie & Co., and Sectoral Asset Management. Series B funds will be used to complete two ongoing U.S. pivotal clinical trials of the Simplify Disc studying its use in one level of the spine and in two adjacent levels of the spine as a treatment for cervical degenerative disc disease, and for commercialization outside of the U.S.
“Having an oversubscribed Series B is a testament to the large opportunity presented by the Simplify Disc,” said Simplify Medical Chief Executive Officer David Hovda. “The Simplify Disc is designed to eliminate the need for CT scans post-surgery, reducing patient risk from associated radiation and solving a significant clinical problem in spine arthroplasty today.”
“We have great confidence in the Simplify Disc and the clinical outcomes achieved to date, and expect the U.S. clinical trials to demonstrate the same,” said Fouad Azzam, Ph.D, with LSP. “Risk mitigation is top of mind for hospitals, and products like the Simplify Disc that maximize patient safety are well aligned with their concerns. In addition, the Simplify Disc offers the lowest-profile device, opening up a broader patient population for the technology.”
“Simplify Medical has developed a very attractive cervical disc replacement system and the company has made tremendous progress on their multiple IDE studies and international commercialization. Our funding will allow them to accelerate these programs and expand access to the Simplify Disc to many more patients,” said Mark Carnegie, founding investor of Simplify Medical.
While magnetic resonance imaging (MRI) is widely used pre-operatively for surgical planning, spine surgeons often switch to CT post-operatively in order to accommodate metal components, which can make it difficult to view the devices, as well as the facets and adjacent disc levels. However, CT scans have been shown to expose patients to ionizing radiation that equates to 400 to 550 chest X-rays per scan.
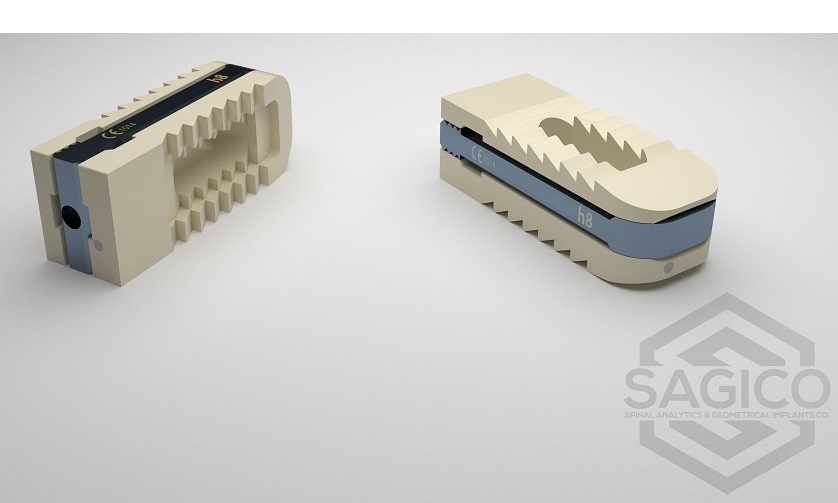
Composed of primarily non-metal materials (PEEK-on-ceramic), the Simplify Disc is designed to be viewed on MRI in order to minimize patient exposure to radiation. With no metal in its articulating components, the disc is also designed for low levels of wear to optimize long-term durability. Implantation of the Simplify Disc is accomplished in a straightforward, three-step procedure. The Simplify Disc is also anatomically designed with low height implant options to accommodate patients with smaller cervical disc spaces, making it ideal for women and certain regional populations. The device is considered MRI-conditional, posing no known hazard in an MRI environment within prescribed conditions of use.
Two Simplify Disc U.S. pivotal trials are currently enrolling. The two-level, prospective pivotal trial will encompass up to 200 patients at up to 18 centers, comparing cervical implantation of the device in two contiguous discs from C3 to C7 with two-level cervical fusion surgery. The other pivotal trial is studying one-level cervical implantation of the device between C3 to C7 compared with one-level cervical fusion surgery. For information about eligibility or enrollment in either pivotal trial, please visit http://www.simplifytrial.com/.
The Simplify Disc has received the CE Mark and is commercially available in select European markets. Early clinical data has shown substantial improvement in patient pain scores and functional improvement after treatment.
ABOUT SIMPLIFY MEDICAL
Simplify Medical is focused on cervical spinal disc arthroplasty, using innovative, MRI-friendly materials designed to decrease the need for ionizing radiation and enhance patient options. Simplify Medical is located in Sunnyvale, California. To learn more, visit http://www.simplifymedical.com/.
ABOUT LSP
LSP (Life Sciences Partners) is an independent European investment firm, providing financing for private and public life sciences companies. LSP’s mission is to connect investors to inventors, focusing on unmet medical needs. With over EUR1.3 billion (USD1.5 billion) of investment capital raised to date and offices in Amsterdam, Munich and Boston, LSP is one of Europe’s leading life sciences investors. In 2017, LSP launched LSP HEF 2 with a fund volume of EUR280 million targeting investment opportunities in private medical technology companies which offer products that can both improve the quality of patient care and lower healthcare spending. To learn more, visit www.lspvc.com.
ABOUT MH CARNEGIE & CO.
M.H. Carnegie & Co. is a leading Australian private equity and alternative asset manager with over AU$500M under management. Carnegie’s investment focus is on high value medical device technologies, with particular emphasis on opportunities that leverage the best innovations, development pathways, management teams and financing strategies. For more information, visit www.mhcarnegie.com.
ABOUT SECTORAL ASSET MANAGEMENT
Sectoral Asset Management is an established global healthcare specialist. Sectoral leverages its expertise and capabilities to capture significant value creation across both public and private companies. More information about the firm and its track record is available at www.sectoral.com.
Caution: The Simplify Disc is an investigational device in the United States and is limited by law to investigational use.
Contacts
Chronic Communications Inc.
Michelle McAdam, (949) 545-6654
michelle@chronic-comm.com