SUNNYVALE, Calif., Sept. 19, 2017 (GLOBE NEWSWIRE) — Relievant Medsystems, a privately held medical device company that has developed the Intracept® Procedure, a minimally invasive and clinically proven approach for the treatment of chronic low back pain, announced today that Kevin Hykes has been appointed President and Chief Executive Officer as well as a member of the Board of Directors, effective immediately. Alex DiNello, the Company’s CEO since 2011, will remain an integral member of the management team as Chief Operating Officer.
Mr. Hykes joins Relievant from Metavention, a clinical-stage medical device company developing interventional therapies for the treatment of type 2 diabetes, where he was Chairman and CEO as well as an Operating Partner at Versant Ventures. He is remaining as Chairman of the Board of Directors at Metavention. Mr. Hykes was previously President and CEO of Cameron Health, developer of the first subcutaneous implantable defibrillator, until its acquisition by Boston Scientific in 2012. Prior to Cameron Health, he was the Chief Commercial Officer of Visiogen, Inc., a privately held ophthalmology company which was acquired by Abbott Medical Optics in 2009. Previously, Mr. Hykes spent sixteen years at Medtronic, where he held leadership positions in the Cardiac Rhythm Management (CRM), Neurological, Heart Valve, and Cardiac Surgery businesses in the U.S. and Europe.
“Kevin is a highly respected industry veteran with an exceptional track record of building and leading strong teams, rapidly growing businesses, and developing markets for groundbreaking, PMA-class devices. Kevin’s experience and track record commercializing medical technologies around the world will be a significant asset as he leads Relievant into the next phase of its growth,” said Richard Mott, Executive Board Chairman of Relievant. “On behalf of the entire Board of Directors, we thank Alex for his contributions which have created a solid foundation for the company’s future success. We look forward to his continued leadership as our Chief Operating Officer.”
“I am thrilled to join Relievant and am impressed by what the company has accomplished to date. The results of the SMART Trial, the medical device industry’s first successful Level 1, randomized sham-controlled trial for chronic low back pain, demonstrate the significant potential for the Intracept Procedure to fill a critical gap in the treatment continuum for chronic low back pain, and to provide physicians and patients with a much-needed new solution,” said Kevin Hykes. “I look forward to working with the Relievant team to deliver on our mission to improve the quality of lives for millions of patients suffering from this disabling condition.”
About Relievant Medsystems
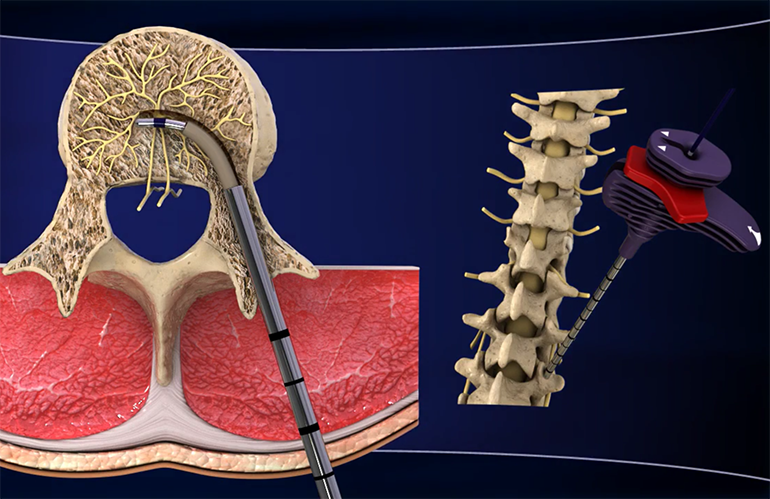
Founded in 2006 and based in Sunnyvale, California, Relievant Medsystems is a privately held medical device company developing new solutions to improve the quality of life for millions of patients suffering from chronic low back pain. Relievant’s Intracept® System delivers targeted energy into the spine and blocks the transmission of pain signals from the basivertebral nerve (BVN). This minimally invasive procedure provides orthopedic surgeons, neurological surgeons and interventionalists with a new way to provide clinically proven, lasting pain relief for chronic low back pain. As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. Please see www.relievant.com for a discussion of the risks, contraindications, warnings, precautions and a summary of the pivotal clinical trial data on the device.
FDA has cleared the Intracept System for the following Indications for Use: The Intracept Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least 6 months duration that has not responded to at least six months of conservative care, and is also accompanied by either Type 1 or Type 2 Modic changes on an MRI.
* Surgical Multi-Center Assessment of RF Ablation for the Treatment of Vertebrogenic Back Pain (SMART)
Contact
Lynn Lewis or Carrie Mendivil
Gilmartin Group
415-937-5402
investors@relievant.com