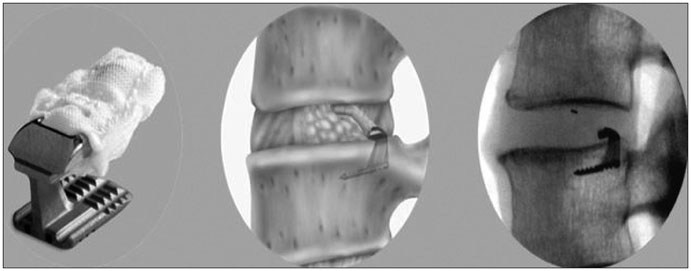
BELGRADE, Mont., May 16, 2017 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE MKT:XTNT), a leader in the development, manufacturing and marketing of orthopedic products for domestic and international markets, today announced it has entered into a licensing agreement with Sites Medical LLC, for utilization of their proprietary OsteoSync™ Ti technology, a best-in-class porous titanium scaffold.
“We are very excited to work with Sites Medical,” said Carl O’Connell, CEO of Xtant Medical. “As market dynamics shift towards emerging titanium technologies, Sites Medical’s OsteoSync Ti technology places us firmly at the forefront of this trend. This technology exhibits great synergy with our entire line of spinal implants further enhancing the value of this relationship as well as Xtant’s potential impact in its core market.”
“We are thrilled to be partnering with Xtant Medical, a company with a rich history of innovation in the spine field,” said Greg Stalcup, President/CEO of Sites Medical. “We look forward to working with the Xtant team to combine our respective technologies to deliver a new generation of high performance, high value products to the market.”
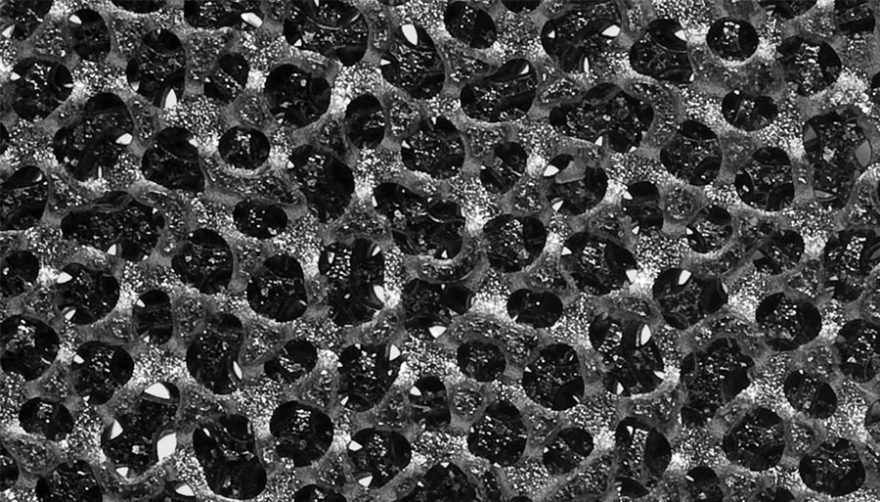
OsteoSync Ti technology is a highly porous titanium scaffold designed to meet the needs of today’s patients from both clinical and economic standpoints. Its high friction coefficient ensures high initial implant stability and its open pore geometry and micro-texturing facilitate superior bone ingrowth. Preclinical testing has demonstrated bone attachment strength nearly twice that of titanium plasma spray and approximately seven times that of PEEK material at the 5-week follow up period, a performance differential that can impact clinical outcomes, especially in spinal fusion patients. OsteoSync Ti technology has also been engineered to reduce the potential for abrasion debris generation during implant insertion, offering an additional measure of safety for the patient. The material is manufactured using highly innovative methods and offers substantial value in today’s cost-conscious healthcare environment.
Utilizing Q2 Metrics data, Xtant Medical estimates the total addressable US market for its technologies that can utilize OsteoSync Ti at $2.5B with a 5 year CAGR approaching 4.75%. Xtant Medical’s first devices to utilize the technology will be featured at this year’s NASS annual meeting to be held on October 25-28 in Orlando, FL.
About Xtant Medical Holdings
Xtant Medical Holdings, Inc. (NYSE MKT:XTNT) develops, manufactures and markets class-leading regenerative medicine products and medical devices for domestic and international markets. Xtant products serve the specialized needs of orthopedic and neurological surgeons, including orthobiologics for the promotion of bone healing, implants and instrumentation for the treatment of spinal disease, tissue grafts for the treatment of orthopedic disorders, and biologics to promote healing following cranial, and foot and ankle surgeries. With core competencies in both biologic and non-biologic surgical technologies, Xtant can leverage its resources to successfully compete in global neurological and orthopedic surgery markets. For further information, please visit www.xtantmedical.com.
About Sites Medical
Sites Medical has recognized the shift in healthcare reimbursement paradigms and is entirely focused on Value-Driven Innovation in orthopedics. With its suite of proprietary orthopedic implant technologies and manufacturing process improvements, Sites aims to deliver improved clinical outcomes and reduced cost to all stakeholders. SITES can further serve the needs of its OEM partners through its Concept-to-Launch capability, where we conduct all necessary R&D activity and use state-of-the-art manufacturing equipment and techniques to deliver the highest quality product. Additional information about the company can be found at www.sitesmedical.com.
Important Cautions Regarding Forward-looking Statements
This press release contains certain disclosures that may be deemed forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to significant risks and uncertainties. Forward-looking statements include statements that are predictive in nature, that depend upon or refer to future events or conditions, or that include words such as “continue,” “efforts,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” “projects,” “forecasts,” “strategy,” “will,” “goal,” “target,” “prospects,” “potential,” “optimistic,” “confident,” “likely,” “probable” or similar expressions or the negative thereof. Statements of historical fact also may be deemed to be forward-looking statements. We caution that these statements by their nature involve risks and uncertainties, and actual results may differ materially depending on a variety of important factors, including, among others: the ability to comply with covenants in the Company’s senior credit facility and to make deferred interest payments; the ability to maintain sufficient liquidity to fund operations; the ability to remain listed on the NYSE MKT; the ability to obtain financing on reasonable terms; the ability to increase revenue; the ability to continue as a going concern; the ability to maintain sufficient liquidity to fund operations; the ability to achieve expected results; the ability to remain competitive; government regulations; the ability to innovate and develop new products; the ability to obtain donor cadavers for products; the ability to engage and retain qualified technical personnel and members of the Company’s management team; the availability of Company facilities; government and third-party coverage and reimbursement for Company products; the ability to obtain regulatory approvals; the ability to successfully integrate recent and future business combinations or acquisitions; the ability to use net operating loss carry-forwards to offset future taxable income; the ability to deduct all or a portion of the interest payments on the notes for U.S. federal income tax purposes; the ability to service Company debt; product liability claims and other litigation to which we may be subjected; product recalls and defects; timing and results of clinical studies; the ability to obtain and protect Company intellectual property and proprietary rights; infringement and ownership of intellectual property; the ability to remain accredited with the American Association of Tissue Banks; influence by Company management; the ability to pay dividends; and the ability to issue preferred stock; and other factors.
Additional risk factors are listed in the Company’s Annual Report on Form 10-K and Quarterly Reports on Form 10-Q under the heading “Risk Factors.” The Company undertakes no obligation to release publicly any revisions to any forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law.
Investor Contact CG CAPITAL Rich Cockrell 877.889.1972 investorrelations@cg.capital Company Contact Xtant Medical Molly Mason mmason@xtantmedical.com