Roswell, GA, April 7, 2017 – Z-Medical LP, subsidiary of Z-Medical GmbH + Co. KG, a privately financed and held medical device company that designs, develops, manufactures, and markets innovative implants and surgical instruments, announced today that it has appointed René Rothacker as Chief Commercial Officer and Jim Talbert as Vice President Sales USA.
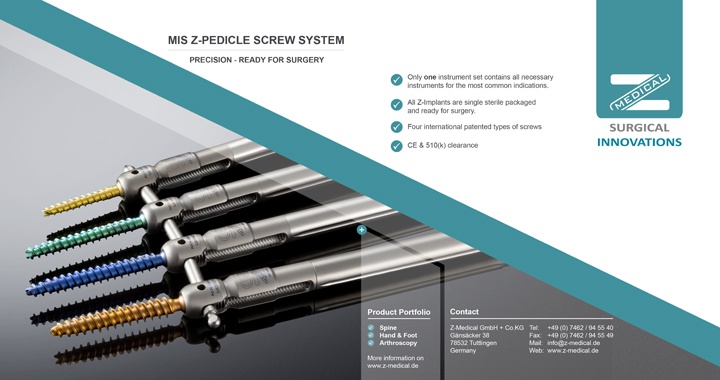
Z-Medical received FDA 510(k) clearance to market the MIS Z-Pedicle Screw System in 1Q15, and established a U.S. subsidiary later that year to support launch with logistic partner WebOps, LCC and GlobalMed Logistix, LLC. In 4Q16, the company entered into an agreement with InterMed Resources tn, LLC to distribute the MIS Z-Pedicle Screw to HealthTrust’s ~1,600 acute care facilities in the U.S.
Mr. Rothacker will report directly to Alexander Henninger, CEO of Z-Medical LP and shareholder of Z- Medical GmbH + Co. KG. René Rothacker is responsible for leading the commercialization of Z-Medical’s MIS Z-Pedicle Screw System including sales and product promotion to drive US business growth.
He has over 15 years of experience in Marketing and Product Management in the medical device industry with an expertise in pharmaceutical packaging and spinal implants. Prior to joining the company, he was Consultant for Z-Medical and provided technical expertise and training in support of product development. Prior to this role he worked as Product Marketing Manager at joimax® GmbH, the leading developer and provider of complete systems for minimally invasive endoscopic spinal surgery and as Senior Product Manager at Paradigm Spine GmbH, a leader in the field of non-fusion spinal implant technology. He was responsible for defining product marketing strategies including preparation of international product launches as well as developing and implementing training programs for sales representatives, global distribution partners and surgeons. From 1999 to 2008, he was employed by Aptar Pharma (former Ing. Erich Pfeiffer GmbH) and was responsible for coordination of international customer projects, product training and support of the global distribution network. 2002, he received his Diploma in Business Administration and Engineering at the University of Cooperative Education at DHBW Stuttgart Campus Horb.
In his previous position as Director International Business Development at Z-Medical he was responsible and the main driver in setting up Z-Medical’s US Operation including the strategic partnership with WebOps, LCC and GlobalMed Logistix, LLC who provide Z-Medical with a comprehensive set of operational processes, tools and services for the US commercialization of MIS Z-Pedicle Screw System.
In this position he has worked in close cooperation with Mr. Talbert who is renowned within the Orthopedic, Neurosurgical, and Spine Industries as a decisive leader and a formidable force in international and regional markets. He formerly was Z-Medical’s USA National Distributor and supported Z-Medical in the start-up phase with his proven history of success in sales and product promotion, with a unique talent for building and maintaining business relationships. He will report to René Rothacker.
The strategic partnership with InterMed Resources tn, LLC who distributes spinal implants manufactured by Z-Medical to HealthTrust Purchasing Group, L.P., a leading group purchasing and total cost management organization that serves nearly 1,600 acute care facilities nationwide was initiated by Jim Talbert. Under the agreement, Z-Medical sells its MIS Z-Pedicle Screw System at a contracted price for distribution to HealthTrust member facilities throughout the United States.
Jim Talbert has extensive experience in training medical professionals in the proper application of surgical equipment and medical devices and is a driven individual committed to developing professional and personal skills to their greatest potential.
His history as a dedicated and organized professional, committed to providing exceptional customer care and support to clients with industry leaders such as Amedica Corp., Exactech, Inc., Globus Medical, Inc., Johnson & Johnson, DePuy, Medtronic, Pfizer, Smith & Nephew, Stryker Corporation, Zimmer, and Zimmer Spine equipped Jim with an expansive knowledge in the surgical device industry.
“We are delighted to have René Rothacker as our CCO and Jim Talbert as VP Sales USA on board of our US team. Their combined expertise and experience in Marketing & Sales will drive our business growth in the US to continue the success in our direct markets.” Alexander Henninger, CEO Z-Medical LP.
“The Innovative Simplicity and high versatility of our MIS Z-Pedicle Screw System can be a game changer, in a technical but also in an economical aspect. It was designed for a straight forward approach and to reduce surgical steps. The preloaded set-screw makes it faster and easier with fewer problems (no cross- threading or tulip splay). The multifunctional system and innovative implant design reduces the OR time, minimizes potential risks and offers a wide range of treatment options.”
About the MIS Z-Pedicle Screw System
The MIS Z-Pedicle Screw System with only one instrument tray offers surgeons an ideal solution for their indication specific needs. The high versatility of the system, the pre-sterilized implants as well as the innovative screw design enables them to efficiently and cost effectively address the most common pathologies. It was specifically designed for a minimally-invasive approach but can also be used in open procedures. It is approved for Degenerative, Trauma, Tumor and Deformity application. Alignment after surgical correction of spondylolisthesis, reduction in fracture- and the derotation in scoliosis treatments are achieved directly with the pre-assembled set-screw, the long reduction thread and the especially designed uniplanar fracture- and deformity screws.
About Z-Medical
Based and founded in Tuttlingen in 2010, Z-Medical® GmbH + Co. KG is a privately financed and held medical device company that designs, develops, manufactures and markets innovative implants and surgical instruments in the section of Spine, Hand & Foot and Arthroscopy. The company’s U.S. subsidiary was established in Atlanta, Georgia, in August 2015. Z-Medical implants stand for precision, innovative simplicity and ready for surgery.
Z-Medical is actively recruiting regional distributors to capitalize on this expansion to its hospital network. For further information about Z-Medical, the MIS Z-Pedicle Screw System or distribution opportunities please contact,
Jim Talbert at j.talbert@z-medical.de
René Rothacker at r.rothacker@z-medical.de
or visit
www.z-medicalUSA.com
Z-Medical LP
1455 Old Alabama Road, East / Suite 115-A Roswell, GA 30076 / USA
Phone: (+1) 404 941 2022
Web: www.z-medicalUSA.com