Ecully, 21 March 2017
Spineway, French specialist in surgical implants and instruments for treating disorders of the spinal column (spine), announces the completion of the first minimally invasive surgery using its Mont-Blanc MIS product line in the United States. The operation was performed by Dr. Ludwig Orozco, neurosurgeon in Dallas (Texas). It is a perfectly demonstrative achievement, given the patient condition was more complex than a typical lumbar osteoarthritis. A recent distribution agreement signed with SLR Medical Consulting was a driving force in this realization.
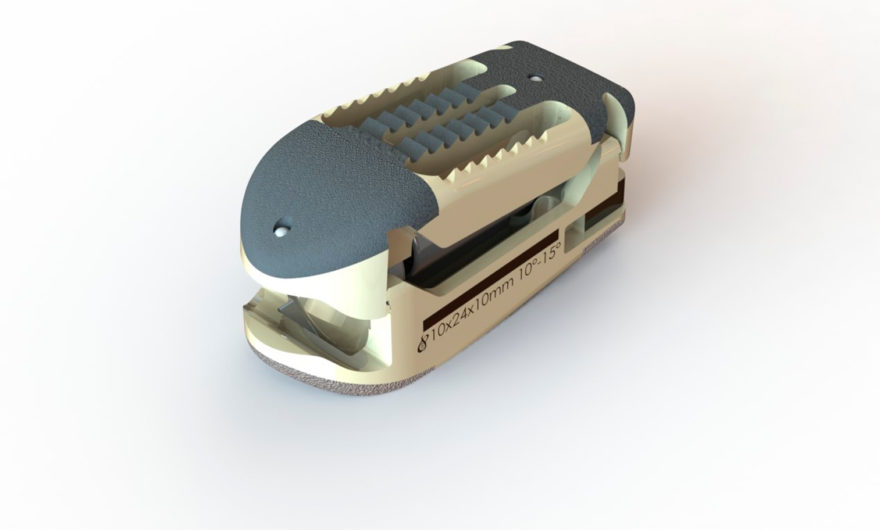
This first implantation of the Mont-Blanc MIS was performed on a patient presenting with degeneration on several vertebral levels. Its surgical treatment required the use of 8 pedicle screws and 2 titanium-alloy rods from Spineway’s innovative Mont Blanc MIS line. These instruments are perfectly adapted to meet practitioners’ growing needs for increasingly technological tools, like constructs involving over 3 vertebral levels, as in the present case. This line provides surgeons with highly technical instruments, all based on Spineway’s expertise in correcting spinal curvatures. The new Mont Blanc MIS instruments already allow for the treatment of several types of lumbar-spine disorders.
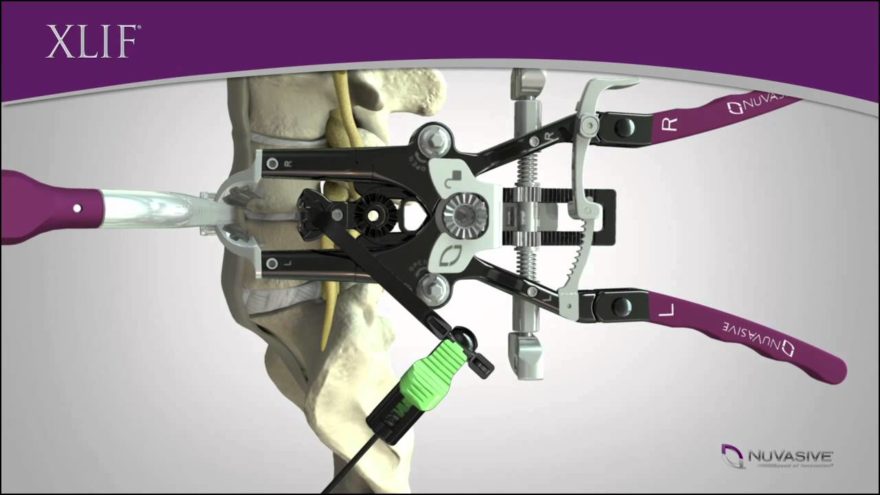
Minimally invasive surgery is currently the most dynamic segment of the worldwide market for spinal implants. Less traumatic for the patient, this type of surgery is less painful, and allows for a faster recovery and shorter hospital stays. This operating technique should, in the medium-term, become the standard technique for most surgeries.
This first successful operation allows Spineway to position itself in the US market on a high-growth segment with an innovative line offering surgeons a multitude of possibilities. Based on the success of its international launch, Spineway is continuing the commercial marketing and deployment of its already highly-technical products and plans to accelerate its R&D in order to widen the scope of application of its technologies, to cover a larger range of disorders.
SPINEWAY IS ELIGIBLE FOR THE PEA-PME (EQUITY SAVINGS PLAN FOR SMES)
Find out all about Spineway at www.spineway.com
Next communication:
2016 Annual Results – 25 April 2017, after market closes
Spineway designs, manufactures and markets innovative implants and surgical instruments for treating severe disorders of the spinal column.
Spineway has an international network of over 50 independent distributors and 90% of its turnover comes from exports.
Spineway, which is eligible for investment through FCPIs (French unit trusts specializing in innovation), received the OSEO Excellence award as well as the Deloitte Fast 50 award in 2011. Rhône Alpes INPI Patent Innovation Award (2013) – Talent INPI award (2015).
ISIN code: FR0011398874 – ALSPW
Contacts:
| Investor Relations David Siegrist – Finance Director Tel: +33 (0)4 72 77 01 52 finance.dsg@spineway.com |
Financial Communication Jérôme Gacoin / Solène Kennis Tel: +33 (0)1 75 77 54 68 skennis@aelium.fr |
Attachments:
http://www.globenewswire.com/NewsRoom/AttachmentNg/5e2e8d19-9523-4a51-896e-71446127fe6b