March 14, 2017
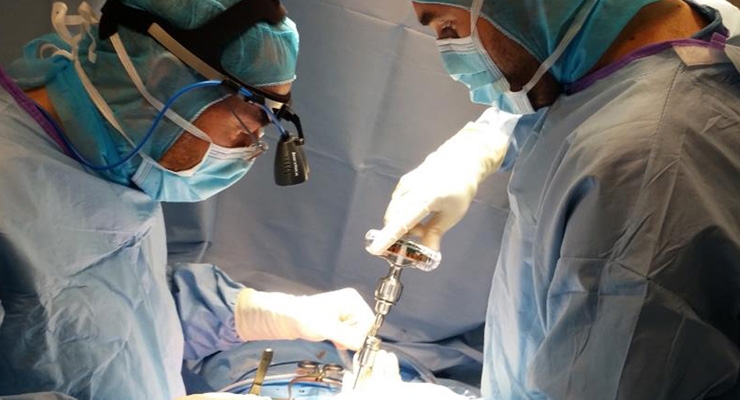
PARIS & SAN FRANCISCO–(BUSINESS WIRE)–SpineGuard (FR0011464452 – ALSGD), an innovative company that develops and markets disposable medical devices to make spine surgery safer, announced today the first cases in the USA using the one-step-insertion of pedicle “smart screws” guided by DSG™ (Dynamic Surgical Guidance) technology. The surgeries were performed successfully by eminent surgeons throughout the USA.
See 3D animation of the “SmartScrew” here.
Thomas Freeman, Professor of Neurosurgery, Tampa, Fla., said: “I knew immediately where the trajectory of the screw was going, even without fluoroscopy. Rather than use five steps to put a screw into the spine, only one step was needed. I could redirect it easily if needed.”
“The DSG technology will change the way spine surgery is performed. It will allow us to place spinal instrumentation faster, safer and with greater accuracy minimizing the risks to our patients,” said Victor Hayes, MD, Orthopedic Surgeon, Trinity Spine Center, Tampa, Fla.
“Integrating the Dynamic Surgical Guidance technology with pedicle screws will greatly optimize the workflow and accuracy, and reduce radiation exposure for surgeons in both the traditional and MIS surgical settings. Not only will the ‘smart screw’ allow for active real-time guidance and breach-avoidance through the pedicle, but it will also provide unprecedented feedback and confidence in the ultimate fixation of the screw itself,” said Larry Khoo, MD, neurosurgeon at The Spine Clinic, Los Angeles, Calif.
“The procedures that we have performed with the self-guiding ‘Smart Screw’ technology have advanced the safety of spinal surgery exponentially by reducing the probability of nerve and spinal cord injury and reducing radiation exposure to patients and hospital staff,”added Farhan Siddiqi, MD, Assistant Professor, University of South Florida, Trinity Spine Center, Florida Advanced Spine, Sports, and Trauma Centers.
John I. Williams, MD, SpineONE, a division of Ortho NorthEast, Ft. Wayne, Ind., concluded: “One-step technology is something that is being explored by multiple implant manufacturers right now…. this ‘smart screw’ with DSG technology is simply going to remove several steps in the process of applying spinal instrumentation.”
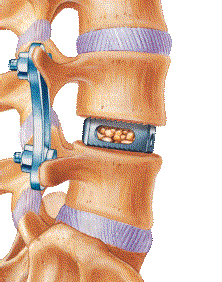
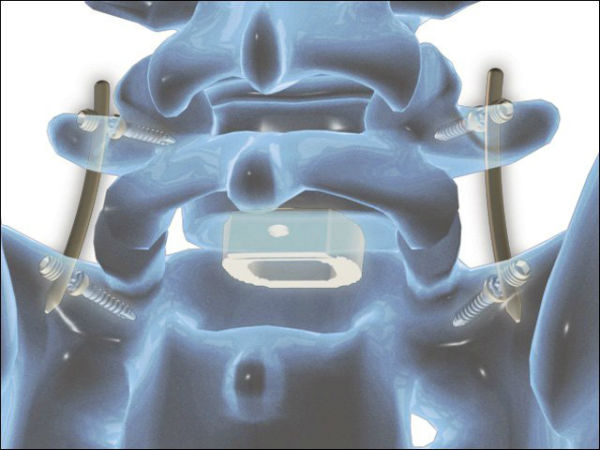
For SpineGuard and Zavation, who announced their co-development partnership in early 2015, these successful first surgeries performed after obtaining 510(k) clearance from the U.S. Food and Drug Administration (FDA) are an essential milestone in preparation for the commercial launch of the Zavation pedicle screw instrumentation that integrates the DSG technology.
Pierre Jérôme, CEO and co-founder of SpineGuard, said: “These surgeries further validate the clinical utility of embedding our DSG technology into the vertebral implant itself, thus enabling its insertion without any preliminary step. We are steadfast in the concept that DSG technology will further secure and simplify the most commonly performed instrumented spine procedure, fusion, while optimizing its cost to the hospital with an ‘integrated guidance’ approach.”
Jeffrey Johnson, CEO and founder of Zavation, added: “Early surgery results with our Z-Direct Screw combined with the DSG technology have been very successful. The single-step technology allows surgeons to use less fluoroscopy and reduce surgery times with excellent screw placement.”
Pedicle screw-based fusion has become the gold standard for treating spine instabilities and deformities. The US spine industry is estimated to have grown 5% between 2015 and 2016 to $7.8 billion in sales to US hospitalsi. This is the highest year-to-year growth in this market since 2010, and reflects the growth in specific sub-segments of the spine market. The US market for spinal implants and devices used in spinal surgery now exceed both the size and growth of the US hip and knee market which was estimated to be $7.5 billion in 2015, up 2.5% from 2014.
More information on the DSG™ technology, its new applications and surgeons’ testimonials here.
More information on the Zavation products that incorporate DSG™ technology click here.
Previous release: SpineGuard extends the utility of its Dynamic Surgical Guidance (DSG ™) technology platform by receiving US patent for “Bone Quality Measurement” application.
Next Press Release: 2016 Full-Year financial results, March 23, 2017
SpineGuard will participate in the ‘Canaccord Genuity Musculoskeletal Conference’ conference on March 14th, 2017 in San Diego, CA before the American Academy of Orthopedic Surgeons Annual Meeting.
About SpineGuard®
Co-founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard’s mission is to make spine surgery safer by bringing real-time digital technology into the operating room. Its primary objective is to establish its proprietary DSG™ (Dynamic Surgical Guidance) technology as the global standard of surgical care, starting with safer screw placement in spine surgery and then in other surgeries. PediGuard®, the first device designed using DSG, was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. It is the world’s first and only handheld device capable of alerting surgeons to potential pedicular or vertebral breaches. Over 50,000 surgical procedures have been performed worldwide with DSG enabled devices. Numerous studies published in peer-reviewed medical and scientific journals have demonstrated the multiple benefits that PediGuard delivers to patients, surgical staff and hospitals. SpineGuard is expanding the scope of its DSG platform through strategic partnerships with innovative medical device companies and the development of smart instruments and implants. SpineGuard has offices in San Francisco and Paris. For further information, visit www.spineguard.com.
About Zavation
Zavation is an employee-owned medical device company that designs, develops, manufactures and distributes medical device products that provide comprehensive medical solutions to improve and enhance quality of life for patients around the world. Zavation is dedicated to exceeding expectations in product quality, customer service, and product cost. For further information, visit www.zavation.com.
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any USA state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
i Source: Orthopedic Network News, 2016 Spinal Surgery Update, Volume 27, Number 4; October 2016.
Contacts
SpineGuard
Pierre Jérôme, +33 (0)1 45 18 45 19
Chief Executive Officer
p.jerome@spineguard.com
or
Manuel Lanfossi
Chief Financial Officer
m.lanfossi@spineguard.com
or
Zavation
Jeffrey Johnson, 601-919-1119
Chief Executive Officer
Jeffrey.johnson@zavation.com
or
Europe / NewCap
Investor Relations & Financial Communication
Florent Alba / Pierre Laurent, +33 (0)1 44 71 94 94
spineguard@newcap.fr
or
US
Ronald Trahan Associates Inc.
Ronald Trahan, APR, +1-508-359-4005, x108