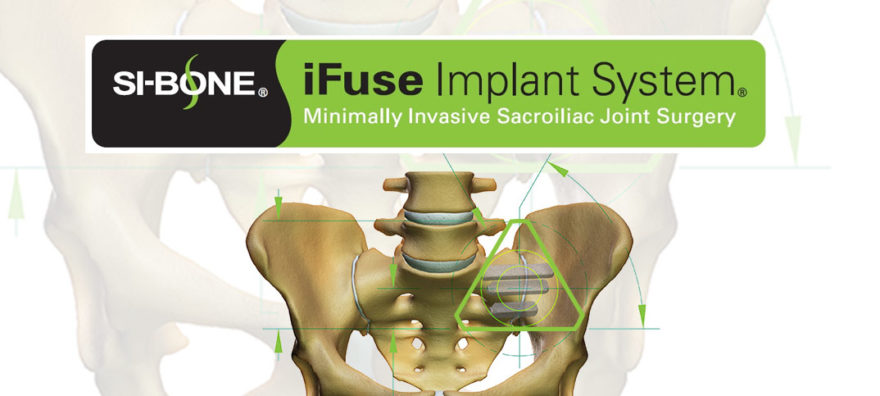
SAN JOSE, Calif., Dec. 15, 2016 /PRNewswire/ — SI-BONE, Inc., an innovative medical device company that pioneered the use of the iFuse Implant System® (“iFuse”), a triangular shaped minimally invasive surgical (MIS) device indicated for fusion for certain disorders of the sacroiliac (SI) joint, announced that Health Care Service Corporation (HCSC), an independent licensee of the Blue Cross and Blue Shield Association (BCBSA), has issued a positive medical policy exclusively for iFuse for MIS SI joint fusion (SUR705.033).
HCSC is the largest customer-owned health insurer in the United States and the fourth largest commercial health insurer overall, with approximately 15 million covered lives insured through Blue Cross and Blue Shield® (BCBS) Plans in Illinois, Montana, New Mexico, Oklahoma, and Texas. The positive coverage policy, which becomes effective January 1, 2017, covers MIS SI joint fusion exclusively using iFuse, based on the large body of published clinical evidence supporting the use of the patented triangular titanium iFuse Implants™ for SI joint fusion. HCSC joins SelectHealth of Utah and Geisinger in Pennsylvania to become the third private insurer to issue a positive coverage policy for MIS SI joint fusion exclusively using iFuse. In addition, the five BCBS plans within HCSC join BCBS of Michigan and BCBS of Nebraska to provide iFuse coverage for BCBS members in seven states.
The policy calls out almost 3 dozen iFuse publications, including two randomized controlled trials (RCTs) comparing iFuse to non-operative care (INSITE and iMIA), long term results from a multicenter prospective single-arm trial (SIFI), as well as data from dozens of additional scientific publications addressing safety, durable effectiveness, biomechanics and cost effectiveness. The policy summary states “based on professional societal organizations, literature reviews, in vitro studies, surveys, and cost-effective studies showing success and/or support for treating SIJ dysfunction with minimally invasive SIJ fusion, including a 2016 retrospective study group of patient surveys greater than 3 years, the evidence is sufficient to determine the effects of the technology on health outcomes. Therefore, minimally invasive SIJ fusion or stabilization, using titanium triangular implants or devices, for the treatment of back pain presumed to originate from the SIJ may be considered medically necessary when meeting all of the specific criteria.” iFuse is the only SI joint fusion device in the U.S. with peer-reviewed publications detailing evidence from prospective trials, and is the only SI joint fusion device with a FDA-cleared indication citing clinical studies that demonstrate improvements in pain, patient function and quality of life.
“The quality and quantity of clinical data supporting iFuse is compelling and was obviously a key element in enabling HCSC to establish exclusive coverage for iFuse,” said Frank Phillips, MD of Midwest Orthopaedics at Rush in Chicago, IL. “Blue Cross Blue Shield patients make up a considerable part of my practice and now, those with SI joint pain caused by SI joint degeneration or disruption will have access to this clinically proven treatment.”
Ralph Rashbaum, MD of the Texas Back Institute in Plano, TX commented: “this is terrific news for Blue Cross Blue Shield patients throughout the state of Texas. I frequently see BCBS patients in my practice who have SI joint pain and now, for those who are properly diagnosed and appropriate surgical candidates, I can offer them the iFuse procedure and eliminate the need for other costly procedures that fail to provide lasting relief of their SI joint symptoms.”
About SI-BONE, Inc.
SI-BONE, Inc. (San Jose, California) is a leading medical device company dedicated to the development, manufacture and commercialization of minimally invasive surgical devices for the treatment of patients with low back symptoms related to certain sacroiliac (SI) joint disorders. SI-BONE, Inc. first received 510(k) clearance to market its iFuse Implant System (“iFuse”) from the Food and Drug Administration (FDA) in November 2008. The CE mark for European commercialization was obtained in November 2010.
The iFuse Implant System provides a minimally invasive surgical solution to fuse the SI joint using patented triangular titanium implants that create an interference fit within the ilium and sacrum. The triangular implant shape, combined with the press fit insertion, is designed to provide immediate fixation by minimizing rotation of the sacrum relative to the illium. The implants have a porous surface that provide an ideal environment for bone ongrowth and ingrowth, facilitating long-term fusion of the joint. iFuse is the only commercially available SI joint fusion system in the United States with published clinical evidence that demonstrates safety, effectiveness and economic benefits, including three large multicenter studies, two of which are randomized controlled trials. Currently, there are more than 45 peer-reviewed publications supporting positive clinical outcomes, safety, biomechanics, and the economic value of iFuse (www.si-bone.com/results). It is the only SI joint fusion system with a FDA clearance recognizing demonstrated improvements in pain, patient function and quality of life following treatment.
The iFuse Implant System is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. Clinical studies have demonstrated that treatment with the iFuse Implant System improved pain, patient function, and quality of life. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit: www.si-bone.com/risks
SI-BONE and iFuse Implant System are registered trademarks of SI-BONE, Inc. ©2016 SI-BONE, Inc. All Rights Reserved. 9751.121516
SOURCE SI-BONE, Inc.
Related Links
http://www.si-bone.com