PORTLAND, Ore.–(BUSINESS WIRE)–Sports Medicine Oregon, a comprehensive orthopaedic clinic treating a wide variety of injuries and illnesses, and Active Implants, a company that develops orthopedic implant solutions, today announced that the first meniscus replacement procedures in Oregon have been performed by orthopaedic surgeons Drs. Richard Edelson and Jonathan Greenleaf. Sports Medicine Oregon and its affiliate Oregon Outpatient Surgery Center is the only site in Oregon – and one of just 10 sites nationwide – enrolling patients with persistent knee pain caused by injured or deteriorating meniscus cartilage in the SUN (Safety Using NUsurface®) clinical trial, which is designed to assess the safety and effectiveness of the NUsurface® Meniscus Implant (pronounced “new surface”) in restoring function similar to that of a natural, healthy meniscus.
The first patient to receive the implant in Oregon was 59-year-old Philomath resident Don Bennett. Bennett grew up playing basketball and initially tore his meniscus in his left knee while playing basketball 30 years ago, only to suffer another tear eight years later. He underwent two meniscectomy surgeries to treat the meniscus tears, but the pain in his knee gradually returned and increased in severity. Over the past five years, the physical demands of his daily job function as a water system maintenance specialist and even daily walking activity aggravated his knee to the point of constant pain.
“There are limited options for patients like Don, who experience persistent knee pain following meniscus injury and surgery,” Dr. Edelson said. “He had exhausted his non-surgical options. Even though his pain had increased in severity over the last five years, he was not a candidate for total knee replacement.”
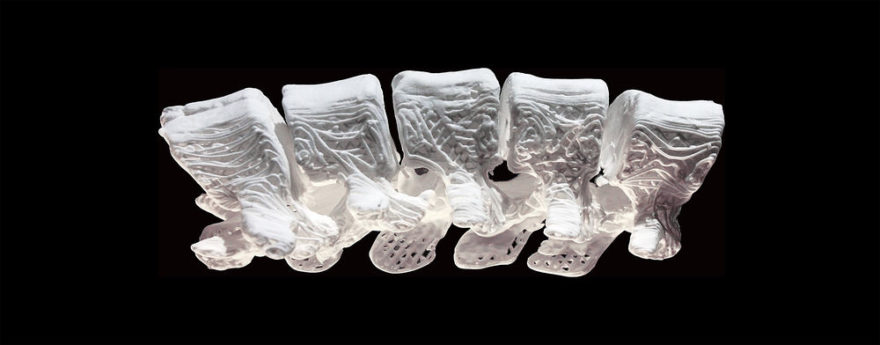
The meniscus is a tissue pad between the thigh and shin bones. Once it is damaged, the meniscus has a very limited ability to heal. Over 1 million partial meniscectomies (to remove the torn portion of the meniscus) are performed in the U.S. every year, more than the total number of hip and knee replacement surgeries combined. However, many patients still experience persistent knee pain following meniscus partial excision surgery.
“Meniscus injuries can be a very painful and debilitating problem, even leading to potential joint replacement at a young age,” Dr. Greenleaf said. “It is our belief that the NUsurface implant may allow patients to delay or avoid knee replacement and return to an improved level of activity without pain.”
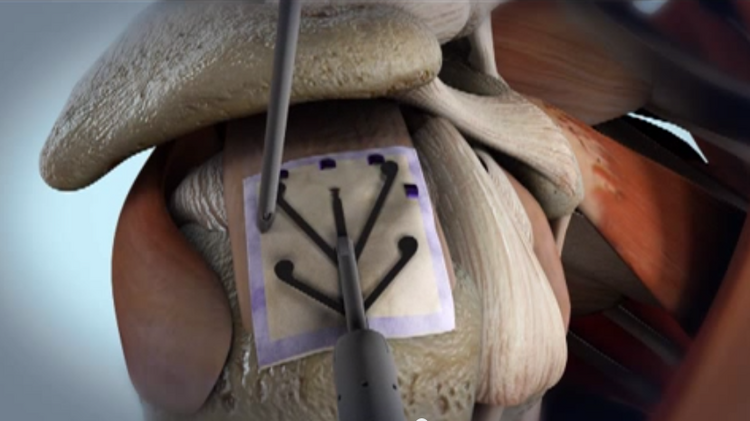
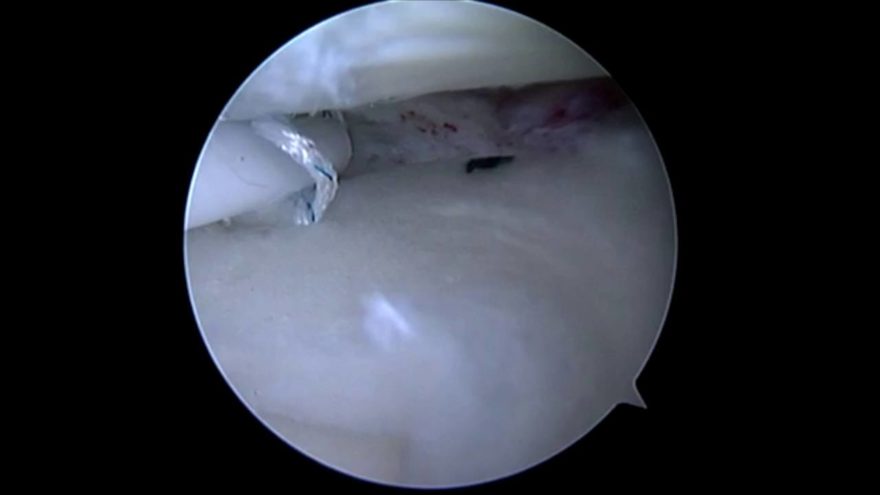
It has been nearly two months since Bennett received the implant on July 13 through a small incision in his knee and completed a six-week rehabilitation program, and he has returned to his daily leisure and sports activities. With only a month left until his retirement from a 35-year career working for the city of Corvallis, he is most looking forward to salmon fishing, spending more time going on walks with his wife, attending his daughter’s Pac 12 volleyball games and coaching his son ahead of his basketball games.
“Prior to receiving the implant, I was living with constant pain and the fear that a total knee replacement was my only option,” Bennett said. “The NUsurface Meniscus Implant is giving me back my mobility. I’m looking forward to supporting my kids’ athletic endeavors, taking hikes with my wife and walking painlessly.”
The NUsurface Meniscus Implant has been used in Europe under CE Mark since 2008 and Israel since 2011.
About the Clinical Trial
The SUN study (Safety Using NUsurface®) will enroll approximately 120 patients as part of regulatory process to gain approval from FDA to sell the device in the U.S. All patients who meet study requirements and agree to enter the trial are offered the NUsurface Meniscus Implant as treatment. Treatment with NUsurface in the SUN trial is eligible for coverage by Medicare and some private insurance companies. To be eligible for the study, participants must be between the ages of 30 and 75 and have pain after medial meniscus surgery that was performed at least six months ago. To learn more about the SUN study, please visit http://sun-trial.com or call (844) 680-8951.
About the NUsurface® Meniscus Implant
The NUsurface® Meniscus Implant, from Active Implants LLC, is an investigational treatment for patients with persistent knee pain following medial meniscus surgery. It is made from medical grade plastic and, as a result of its unique materials, composite structure and design, does not require fixation to bone or soft tissues. The NUsurface Meniscus Implant mimics the function of the natural meniscus and redistributes loads transmitted across the knee joint. Clinical trials are underway in the U.S., Europe and Israel to verify the safety and effectiveness of the NUsurface Meniscus Implant.
About Sports Medicine Oregon
The goal of Sports Medicine Oregon is the restoration of active lifestyles to injured athletes of all ages. The team of specialty-trained physicians takes pride in providing the community with the highest quality orthopedic care. Whether it is the professional athlete or the weekend warrior, it strives to provide timely and comprehensive care at the state-of-the-art facility which includes full service X-ray, outpatient physical therapy, and the adjoining surgery center. For more information, please visit http://sportsmedicineoregon.com/.
About Oregon Outpatient Surgery Center
Oregon Outpatient Surgery Center is a multispecialty surgical center, which includes joint replacements. Oregon Outpatient Surgery Center provides caring, friendly, highly-skilled and trained professional staff, delivering the personal attention every patient deserves in a comfortable, professional atmosphere, a cost effect alternative to traditional surgical care.
About Active Implants
Active Implants, LLC develops orthopedic implant solutions that complement the natural biomechanics of the musculoskeletal system, allowing patients to maintain or return to an active lifestyle. Active Implants is privately held with headquarters in Memphis, Tennessee. European offices are in Driebergen, The Netherlands, with R&D facilities in Netanya, Israel. For more information, visitwww.activeimplants.com.
CAUTION Investigational device. Limited by United States law to investigational use.
Note to Editors: Photos of Don Bennett and Drs. Richard Edelson and Jonathan Greenleaf are available upon request.