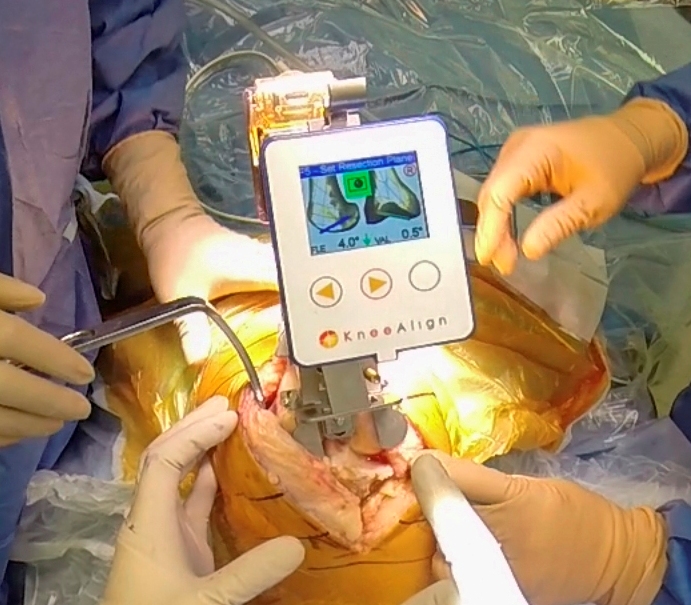
NEWPORT BEACH, CA–(Marketwired – Jun 14, 2017) – ORHub, Inc. (OTC: ORHB), a cloud-based medical software company established as the Operating Room Hub for data and analytics, today announces that it is launching its new service module for hip and knee surgical procedures, July 2017. With over 100 million surgeries performed in the U.S. every year and growing, this new service line significantly expands the addressable market for the Company’s dynamic analytics capabilities and Surgical Resource Management platform.
“The launch of this new module follows the successful launch of our spine module, which for the past eight months has been in continuous use in two nationally recognized hospitals, achieving overwhelming success. Our hips and knees module is the second of many on our technical road map that will allow us to exponentially grow our addressable market and take advantage of the resulting opportunities,” says ORHub CEO Colt Melby.
According to the Agency for Healthcare Research and Quality, more than 300,000 total hip replacements and 600,000 knee replacements are performed each year in the United States, a figure expected to grow by 13.19 % per year for the next five years. ORHub’s hip and knee service module presents to this growing market unprecedented procedure data and advanced analytics.
ORHub’s Surgical Resource Management software transforms the OR into a highly efficient information center that provides the ability to track and monitor surgical implants, compare material costs, and identify physician and financial impacts in the operating room at the point of surgery. The software also tackles the data chaining needed for cost, usage and eventual outcome correlation in all surgeries without heavy IT investments in infrastructure.
The Company’s spine service line is currently being used for all spine cases in the operating room of two major hospitals in Southern California, where the company recently announced it has successfully collected data from more than 500 surgeries (http://nnw.fm/DTkw3), a number that continues to grow on a daily basis. The data collected provides the hospitals valuable insight that helps identify user needs and make near real-time data driven decisions to improve profitability and the quality of patient care.
“We look forward to updating shareholders on our progress as we continue to build new service modules at a rapid pace conducive to increased corporate value,” says Melby.
ORHub, Transforming the Business of Surgery
The ease of deployment is weeks instead of years, and the power to provide process data for illumination of costs and improvement, gives OR directors and hospital administrators powerful new capabilities to transform the business of surgery. ORHub is capable of agile, low-cost integration with a hospital’s system of choice to complement existing capabilities and provide analytics that are currently not available in the OR suite as a comprehensive view of each episode of care. This puts decision making power at the fingertips of directors and administrators as well as physicians and vendors.
Its case-based subscription model also transforms the business of surgery by moving the infrastructure costs from a CAPEX budget cycle into the operational budget of the OR director. A hospital’s time to insight is transformed from months or weeks to minutes, and the revenue cycle is compressed so time to money is quicker for substantial cash flow improvement.
About ORHub, Inc.
ORHub is a cloud-based software company focused on delivering performance-based medicine at the speed of surgery. The system is in full operation and in daily use at two major hospitals in Southern California.
ORHub enables all parties involved in surgical care to work together to organize, deliver, measure and reimburse in a single uniform process. ORHub’s cloud capabilities also provide a bridge for hospitals in their hybrid cloud strategies, integrating with EHRs and other systems as requested. ORHub is an agile answer for hospitals looking to unlock the power of the OR. Its case-based subscription revenue model also transforms the economics of hospital software from a CAPEX to an OPEX budgeting process.
The need for ORHub is clear. Health care comprises more than 17% of US GDP at over $3 trillion per year. With costs rising every year due to an aging population and more expensive treatments, providers are under severe pressure to become more efficient and reduce costs from payers who are aggressively reducing reimbursements and moving away from fee-for-service and toward performance-based reimbursement. ORHub enables providers to thrive in this new environment by addressing the single largest segment of health care, which is surgical care. ORHub replaces numerous legacy systems with a 360-degree system that is focused on tracking cost from diagnosis to discharge. ORHub has offices in Phoenix, Arizona; Newport Beach, California; and Bellevue, Washington.
Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Although the Company believes the expectations reflected in any forward-looking statements are based on reasonable assumptions, it can give no assurances that its expectations will be attained. Such statements are inherently uncertain, and actual results and activities may differ materially from those estimated or projected. Certain factors that can affect the Company’s ability to achieve its anticipated results include, among others, uncertainties inherent in the development of a new software product business.
CONTACT INFORMATION
- Communications Contact:
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Email Contact