16 May 2017
Smith & Nephew (LSE:SN, NYSE:SNN), the global medical technology business, proudly supports results of an independent, randomised clinical trial1 concluding that, in the patients studied, the use of the PICO Single Use Negative Pressure Wound Therapy (NPWT) system significantly reduced the rate of surgical site infections (SSI) by 74%, compared to standard care in patients undergoing major abdominal incisions.
The randomized, controlled, open-label trial of 50 patients investigated the role of PICO Single Use NPWT used prophylactically in patients undergoing major abdominal surgery. Thirty days after operations, the incidence rates of SSI significantly reduced by 74% (8.3% in treatment group vs. 32% in control group). Patients’ length of stay also reduced by approximately eight days (6.1 vs. 14.7 days). The treatment group included the use of the PICO Single Use NPWT system.
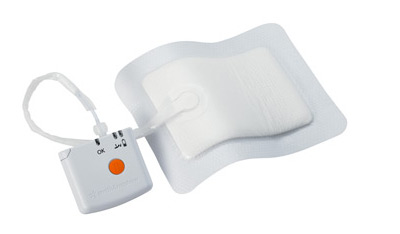
Patients at risk of poor healing may benefit from the PICO Single Use NPWT System, as it can help to improve the speed, strength, and quality of incisional wound closure, and may minimise the failures of healing that may lead to infection and/or dehiscence2. The PICO system is suitable for use in both a hospital and community setting and approved for a number of indications, including surgically closed incision sites.
“This study underscores the importance of PICO Single Use NPWT in treating patients who have undergone a laparotomy (open abdominal surgery),” said Colin Peirce, Consultant General and Colorectal Surgeon, University Hospital Limerick, Ireland. “As surgeons, we are always looking for effective and cost efficient ways to treat patients, and this study demonstrates that PICO Single Use NPWT can significantly reduce both the incidence of surgical site infection and the length of stay, resulting in a potential reduction in healthcare costs,” concludes Colin Pierce.
Up to 60% of all SSIs are preventable but they continue to be a large burden on the healthcare system3. With approximately 500,000 SSIs per year in the US and 8,000 connected annual deaths, the cost of SSIs are in excess of $7 billion and over £758 million per year in the UK3.
“This study is the latest addition of research that reinforces the importance of PICO Single Use NPWT and the significant impact it has on reducing SSIs, healthcare costs and ultimately improving the patient’s quality of life,” said Andy Weymann, Chief Medical Officer in Smith & Nephew. “It follows the recent release of global guidelines from the World Health Organisation (WHO) recommending the use of NPWT prophylactically,” added Andy Weymann.
The PICO Single Use NPWT system is being investigated in a number of clinical trials worldwide. For more information about the clinical trials, please visit: www.clinicaltrials.gov.
Enquiries
Media
Karley Ura
Madano
+44 (0) 20 3595 2415
Jaclyn Confalone
Madano
+44 (0) 20 3595 2441
Dave Snyder
Smith & Nephew
+1 (978) 749-1440
About Negative Pressure Wound Therapy (NPWT)
Negative Pressure Wound Therapy (NPWT) has been in use for more than 20 years for the management of a wide range of different wound types in adults, including traumatic hard-to-heal and chronic wounds, and wounds covered with flaps and/or skin grafts. It has also been used for the management of complex wounds. More recently, NPWT systems have been used to manage closed surgical incisions in patients at high risk of surgical site complications.
About Smith & Nephew
Smith & Nephew is a global medical technology business dedicated to helping healthcare professionals improve people’s lives. With leadership positions in Orthopaedic Reconstruction, Advanced Wound Management, Sports Medicine and Trauma & Extremities, Smith & Nephew has around 15,000 employees and a presence in more than 100 countries. Annual sales in 2016 were more than $4.6 billion. Smith & Nephew is a member of the FTSE100 (LSE:SN, NYSE:SNN).
For more information about Smith & Nephew, please visit our website www.smith-nephew.com, follow @SmithNephewplc on Twitter or visit SmithNephewplc on Facebook.com.
To learn more about what we do to help reduce surgical site complications, please visit www.closertozero.com.
Reference:
- O’Leary, D.P. et al, Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations. A randomized, controlled, open-label trial: The PICO Trial. Annals of Surgery, published online 06 December 2016.
- S. Karlakki, M. Brem, S. Giannini, V. Khanduja, J. Stannard, R. Martin. Negative pressure wound therapy for management of the surgical incision in orthopaedic surgery. Bone Joint Res 2013;2:276–84.
- World Union of Wound Healing Societies (WUWHS) Consensus Document. Closed surgical incision management: understanding the role of NPWT. Wounds International, 2016
Forward looking statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as “aim”, “plan”, “intend”, “anticipate”, “well-placed”, “believe”, “estimate”, “expect”, “target”, “consider” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith & Nephew, these factors include: economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith & Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith & Nephew’s most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith & Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith & Nephew are qualified by this caution. Smith & Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith & Nephew’s expectations.
™ Trademark of Smith & Nephew. Certain marks registered US Patent and Trademark Office