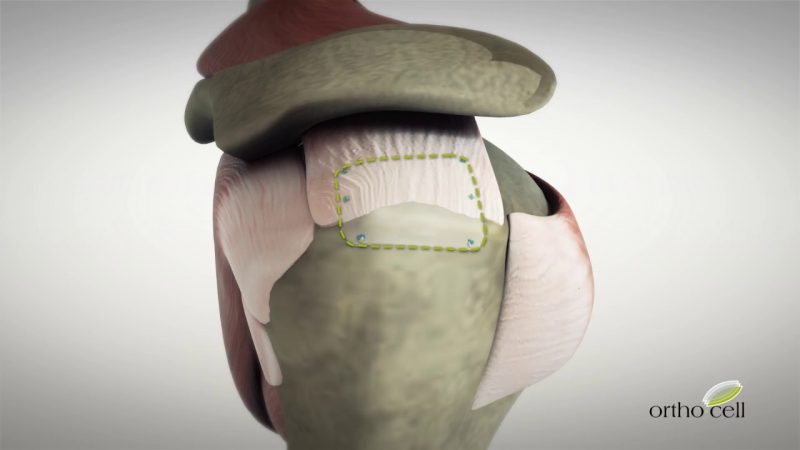
NAPLES, Fla., Jan. 19, 2018 /PRNewswire/ — Catalyst OrthoScience, Inc. has been granted four new patents by the United States Patent and Trademark Office for technologies incorporated in the CSR™ Total Shoulder Replacement System. The Catalyst CSR™ Total Shoulder System is a new canal-sparing, anatomic shoulder arthroplasty system that provides consistently reproducible shoulder joint restoration with an humeral implant that is smaller, more anatomically-shaped, and less invasive than traditional shoulder replacement surgery.
|
U.S. Patent Number |
Title |
Patent Issue Date |
|
9,814,588 |
Glenoid Arthroplasty with Multi-directional Fixation |
November 14, 2017 |
|
9,814,587 |
Humeral Arthroplasty |
November 14, 2017 |
|
9,814,471 |
Glenoid Arthroplasty with Offset Reamers |
November 14, 2017 |
|
9,775,716 |
Glenoid Arthroplasty |
October 3, 2107 |
These patents expand the portfolio of Catalyst intellectual property related to the Company’s proprietary glenoid and humeral implant designs and instrumentation. “The granting of these patents endorses the extent of our technology and provides Catalyst with further protection for its product offerings in the shoulder replacement market,” said Bob Kaufman, CEO of Catalyst OrthoScience. “We have built a solid intellectual property portfolio and will continue to work to extend the depth and breadth of that protection.”
With core technology driven by surgeon and patient need, Catalyst has been able to draw a loyal following of orthopedic surgeons since receiving FDA clearance for the CSR™ total shoulder replacement solution last year. “Catalyst’s core technology is truly innovative, as evidenced by not only the growing patent portfolio, but also by the growing number of high caliber shoulder specialists who have embraced Catalyst as an important part of their clinical offering,” said Rod Allen, Catalyst’s Senior Vice President of Sales and Marketing.
Catalyst OrthoScience was founded in 2014 by orthopedic surgeon Steven Goldberg, M.D. who realized, based on his own experience as a fellowship-trained shoulder specialist, that improvements were needed to make shoulder replacement surgery less invasive and to give patients a more normal feeling shoulder after surgery. “It feels great to be granted these patents,” said Dr. Goldberg. “I’m still amazed at how much our team has accomplished in such a short timeframe, and now patients are seeing the benefit.”
About Catalyst OrthoScience Inc.
Headquartered in Naples, FL, Catalyst OrthoScience develops and markets innovative medical device solutions that make orthopedic surgery less invasive and more efficient for both surgeons and patients.
The company’s first offering is the Catalyst CSR Shoulder System. The Catalyst CSR is a single-tray total shoulder arthroplasty system containing a non-spherical humeral implant for consistent anatomic joint line restoration and specialized glenoid instrumentation for a less invasive approach that preserves the natural anatomy and removes less of the patient’s bone.
Catalyst OrthoScience’s products are marketed under a portfolio of brands including Catalyst OrthoScience® and Catalyst CSR™. For additional information on the Company, please visit http://www.catalystortho.com.
SOURCE Catalyst OrthoScience, Inc.