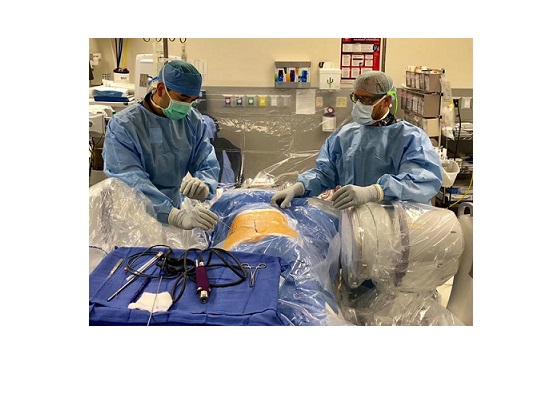
RESTON, Va., Oct. 10, 2018 (GLOBE NEWSWIRE) — Dr. Colin Haines, assisted by Dr. Christopher Good, Spine Surgeons at Virginia Spine Institute, is the first physician to perform endoscopic spine surgery at Reston Hospital Center. Through a micro incision (less than 1cm), Dr. Haines and Dr. Good utilized the magnification of an HD-endoscope to perform a microdiscectomy. This surgical method allowed safer and more efficient removal of a disc herniation.
Minimally invasive procedures are now commonplace in spinal surgery, but endoscopic spine surgery utilizes ultra-minimally invasive technology that enhances traditional surgical technique. The advantage of a smaller incision (less than 1cm) and the introduction of a high-resolution camera minimizes tissue disruption and reduces post-operative pain for the patient. Typically performed as an outpatient procedure, patients undergo less anesthesia and experience improved recovery times.
A wide-reaching innovation, endoscopic spine surgery has promise to transform the lives of patients with degenerative disc disease, disc herniations, and spinal stenosis. This milestone is an essential breakthrough for future spinal procedures as the specialists at Virginia Spine Institute work to advance endoscopic technology for spinal fusions and spinal decompressions.
“Endoscopic spine surgery has unleashed new potential for faster, safer, and more effective surgical recoveries,” says Dr. Colin Haines, Spine Surgeon and Director of Research at Virginia Spine Institute. “I’m proud to dramatically improve the lives of patients suffering from back pain by pioneering treatment solutions like this that get them back to their active lifestyles faster.”
Dr. Colin Haines is an expert in minimally invasive surgical techniques that improve patient safety and reduce recovery time. These techniques include ultrasonic spine surgery, endoscopic spine surgery, and robot-guided spine surgery.
Dr. Christopher Good, Spine Surgeon and President of Virginia Spine Institute, specializes in the treatment of complex spinal conditions and is considered a world expert in the field of robotic spine surgery.
About Virginia Spine Institute: Virginia Spine Institute is an award-winning specialty medical practice in the Washington, DC metro area solely dedicated to spinal health care. For over 25 years they have improved the lives of over 85,000 patients suffering from back or neck pain conditions. This comprehensive spine center provides convenience of specialty care with the top experts from multiple specialties of spinal health care under one roof. Custom treatment options include non operative care, pain management, physical therapy, and when necessary surgical intervention, including minimally invasive, laser and robot-guided procedures. For more information about Virginia Spine Institute visit SpineMD.com.
Photo accompanying this announcement are available at
http://www.globenewswire.com/NewsRoom/AttachmentNg/71cbbbb0-a542-4be7-9ca8-c8047319cddf
http://www.globenewswire.com/NewsRoom/AttachmentNg/b8e761d3-ef29-4ea3-acb7-c71cdc88752b
Contact: Communications Director Virginia Spine Institute Tel: 703.709.1114 x 180 Email: SpineMD.com