CARLSBAD, Calif., April 07, 2017 (GLOBE NEWSWIRE) — Alphatec Spine, Inc., a wholly owned subsidiary of Alphatec Holdings, Inc. (Nasdaq:ATEC) and a provider of spinal fusion technologies, announced today that the Company has launched its new Battalion Lateral System with the Alphatec Squadron Lateral Retractor, and successfully completed initial patient surgeries including degenerative, multilevel and L4/L5 spinal segment cases. With the launch of the Battalion Lateral System, the Company is well positioned to begin to compete in the $500M U.S. Lateral market.
“The launch of Battalion Lateral represents a significant milestone for Alphatec, opening up new commercial opportunities for us. With this launch, we are now able to compete in the MIS Lateral market—one of the fastest growing markets in spine,” said Terry Rich, Alphatec Spine’s Chief Executive Officer. “The Battalion Lateral System includes our proprietary Squadron Retractor that is designed to enhance the surgeon’s experience and improve clinical outcomes. Early feedback from surgeon customers has been very positive regarding the system performance, differentiated feature set and ability to successfully treat even the most complex patient cases with a minimally invasive approach. The launch of Battalion Lateral also enables Alphatec to access new distributors with strong surgeon relationships in the Lateral space. We look forward to expanding into this new market and increasing surgeon adoption.”
Battalion Lateral System Overview
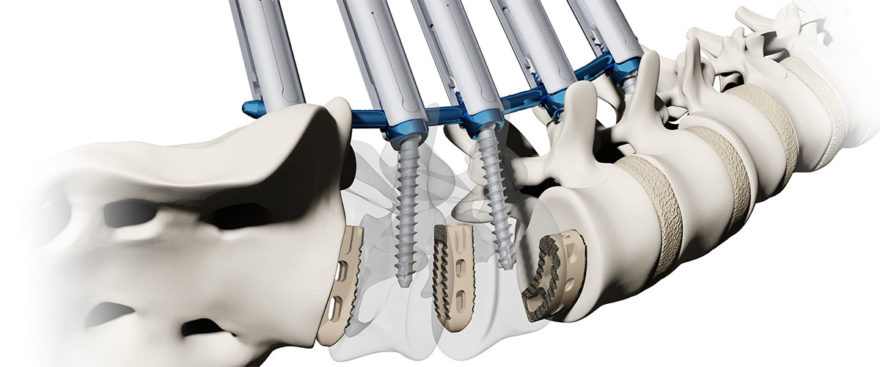
The Battalion Lateral System with the Alphatec Squadron Lateral Retractor provides surgeons with a next-generation Lateral system with innovative, unique functionality designed to improve clinical outcomes by reducing tissue creep, minimizing psoas retraction time, and achieving alignment and fusion objectives. The Battalion Lateral System includes numerous proprietary features, including the Squadron Lateral Retractor. The system is designed to allow surgeons to customize the access to match the patient’s unique anatomy through independent retraction of the cranial/caudal blades, DepthControl™ technology that provides in-situ height adjustment for the low-profile blades, and LevelToe™ mechanics to ensure that the blades maintain a parallel plane when toed up to 15°. The Squadron Retractor is also fully compatible with most neuromonitoring platforms enabling access safely through the psoas. The Battalion Lateral Spacer is available in 0° and 15° lordosis with a variety of width and height options for lumbar and thoracic approaches as well as angled and offset instrumentation to provide access to the L4/L5 segment.
“Alphatec’s Battalion Lateral System provides great options for accessing and preparing the space via the retractor, but the retractor itself completes the procedure,” said Dr. Frank K. Kuwamura, a board-certified orthopedic spine surgeon, in San Antonio, Texas. “The ability to independently raise and lower blades to accommodate the anatomy really separates this retractor from other retractors available on the market. It saves time in the psoas and that supports better patient outcomes.” Dr. Kuwamura was one of the first surgeons to use the system and completed the case with Alphatec’s Illico® percutaneous pedicle screws. The patient had a previous fusion and had developed adjacent disc disease.
Michael E. Russell, II, M.D., a board-certified orthopedic surgeon in Tyler, Texas, was also one of the first surgeons to use the Battalion Lateral System in a clinical setting. He used the system to perform a Lateral procedure at L3/L4 and instrumented posteriorly using Alphatec’s Arsenal™ Spinal Fixation System. The Squadron Lateral Retractor allowed Dr. Russell to access the disc space from an offset trajectory.
Dr. Russell commented, “The Squadron Retractor enabled me to attach to multiple attachment points giving me the flexibility to use my preferred Lateral technique. The combination of the level toeing and the ability to lower the low-profile blades individually allowed me to successfully navigate osteophytes without the need for blade extenders.”
Battalion Lateral to be Featured at Upcoming Surgeon Conferences
The Battalion Lateral System and Squadron Retractor will be featured at the International Society for the Advancement of Spine Surgery, ISASS, April 12th through April 14th in Boca Raton, Florida and at the Annual Meeting of the American Association of Neurological Surgeons, AANS, April 24th through April 26th in Los Angeles, California for surgeon review.
About Battalion Lateral System and Squadron Retractor Lateral Access System
The Battalion Lateral System features a full array of access and disc preparation instrumentation in straight, angled, and offset orientations. The system is recommended for use with the Arsenal Spinal Fixation System or the Illico MIS Posterior Fixation System. The Battalion Lateral implant is also cleared for use with both autograft and allograft biologic materials.
For more information, please visit: http://alphatecspine.com/Battalion-Lateral.
About Alphatec Spine
Alphatec Spine, Inc., a wholly owned subsidiary of Alphatec Holdings, Inc., is a medical device company that designs, develops and markets spinal fusion technology products and solutions for the treatment of spinal disorders associated with disease and degeneration, congenital deformities and trauma. The Company’s mission is to improve lives by delivering advancements in spinal fusion technologies. The Company markets products in the U.S. via independent sales agents and a direct sales force.
Additional information can be found at www.alphatecspine.com.
Forward Looking Statements
This press release may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainty. Such statements are based on management’s current expectations and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Alphatec Spine cautions investors that there can be no assurance that actual results or business conditions will not differ materially from those projected or suggested in such forward-looking statements as a result of various factors. Forward-looking statements include the Company’s ability to compete and expand its presence within the U.S. MIS Lateral market and the size of such market; the Company accessing new distributors with strong surgeon relationships in the Lateral space and increasing surgeon adoption; and the ability of Battalion™ Lateral System to enhance the surgeon’s experience and improve clinical outcomes. The words “believe,” “will,” “should,” “expect,” “intend,” “estimate” and “anticipate,” variations of such words and similar expressions identify forward-looking statements, but their absence does not mean that a statement is not a forward-looking statement. The important factors that could cause actual operating results to differ significantly from those expressed or implied by such forward-looking statements include, but are not limited to: the uncertainty of success in developing and launching new products, including without limitation the products discussed in this press release; the Company’s ability to compete directly with the market leaders in the U.S. MIS Lateral market; the Company’s ability to gain market share in the U.S. MIS Lateral market and to benefit a vast number patients in such market; and the acceptance of the Company’s products by the surgeon community and the success of procedures by spine surgeons, including without limitation the products and procedures discussed in this press release. Please refer to the risks detailed from time to time in Alphatec Holdings’ SEC reports, including its Annual Report Form 10-K, as well as other filings on Form 10-Q and periodic filings on Form 8-K. Alphatec Spine disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, unless required by law.
CONTACT: Investor/Media Contact: Christine Zedelmayer Investor Relations Alphatec Spine, Inc. (760) 494-6610 czedelmayer@alphatecspine.com