SAN JOSE, Calif., April 3, 2017 /PRNewswire/ — SI-BONE, Inc., an innovative medical device company that pioneered the use of the iFuse Implant System® (“iFuse”), a triangular shaped minimally invasive surgical (MIS) device indicated for fusion for certain disorders of the sacroiliac (SI) joint, announced that the highly respected journal Spine has published the 50th peer-reviewed iFuse paper titled Predictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint – a Pooled Analysis1. Compared to analyses performed in individual trials, pooling data from these three trials allowed a more statistically powerful determination of patient factors that could predict clinical outcomes after either surgery or non-surgical treatment for appropriately diagnosed patients with SI joint dysfunction.
The pooled analysis study included 423 patients from the three combined multicenter prospective trials, two of which were randomized controlled trials, in which 97 patients received non-surgical management (“NSM”) and 326 patients received SI joint fusion with the iFuse Implant™ from 2013 to 2015. Overall, positive effectiveness, durability and opioid user reduction responses were much higher in the iFuse Implant group compared to the NSM group. In the NSM group, there were no predictors of improved outcomes. In contrast, in the iFuse Implant group, smoking and opioid use were predictive of somewhat smaller improvements in pain relief and disability whereas higher patient age and longer duration of pain were predictive of larger improvements. Although statistically significant, the difference in treatment responses in these groups were clinically unimportant; that is, all subgroups had clinically large improvements after SI joint fusion with iFuse Implants.
“These results indicate that SI joint fusion with triangular iFuse Implants leads to better treatment outcomes compared to non-surgical management and that the extent of improvement with SI joint fusion is only modestly associated with smoking, opioid use, patient age and duration of pain,” said Daniel Cher, MD, Vice President of Clinical Affairs at SI-BONE and study co-author. “Some surgeons do not offer some treatments to smokers or opioid users; for SI joint fusion with iFuse Implants, however, smokers and opioid users had marked and clinically important responses.”
The company also announced that the iFuse Implant System has been used in more than 25,000 procedures worldwide and continues to be the Method of Choice for SI Joint FusionSM. Adoption of the iFuse Procedure™ has continued to grow as surgeons learn to include the SI joint as part of their routine low back pain diagnostic exam and insurance coverage for the procedure has expanded. Recently, Healthcare Service Corporation (HCSC), the 4th largest commercial health plan in the U.S., established an exclusive coverage policy for iFuse for Blue Cross Blue Shield participants in Texas, Illinois, Montana, New Mexico and Oklahoma.
“These are two remarkable milestones that were made possible with over eight years of relentless dedication by our organization and thousands of health care providers who manage patients with SI joint disorders,” said Jeffrey Dunn, President and CEO of SI-BONE. “Together, we have raised awareness and educated thousands on SI joint diagnosis and treatment and with continued focus on education and clinical evidence, we are hopeful that all those suffering from chronic SI joint dysfunction who fail conservative care are able to obtain the appropriate therapy to help them.”
About SI-BONE, Inc.
SI-BONE, Inc. (San Jose, California) is a leading innovative medical device company dedicated to the development, manufacture and commercialization of minimally invasive surgical devices for the treatment of patients with low back symptoms related to certain sacroiliac (SI) joint disorders. SI-BONE, Inc. first received 510(k) clearance to market its iFuse Implant System (“iFuse”) from the Food and Drug Administration (FDA) in November 2008. The CE mark for European commercialization was obtained in November 2010.
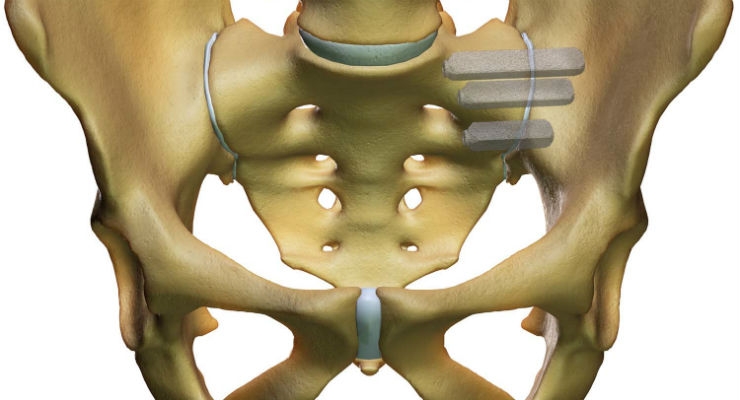
The iFuse Implant System provides a minimally invasive surgical solution to fuse the SI joint using patented triangular titanium implants that create an interference fit within the ilium and sacrum. The triangular implant shape, combined with the press fit insertion, is designed to provide immediate fixation by minimizing rotational motion. The implants have a porous surface that provide an ideal environment for bone ongrowth and ingrowth2, facilitating long-term fusion of the joint. The iFuse Implant is the only commercially available SI joint fusion device in the United States with significant published prospective clinical evidence that demonstrates safety, effectiveness and economic benefits, including three large multicenter studies, two of which are randomized controlled trials. Currently, there are 50 peer-reviewed publications supporting positive clinical outcomes, safety, biomechanics, and the economic benefits of the iFuse Implant (www.si-bone.com/results).
The iFuse Implant System is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit: www.si-bone.com/risks
SI-BONE and iFuse Implant System are registered trademarks of SI-BONE, Inc. ©2017 SI-BONE, Inc. All Rights Reserved. 9758.040317
- Dengler J, Duhon B, Whang P, Frank C, Glaser J, Sturesson B, Garfin S, Cher D, Rendahl A, Polly D, on behalf of the INSITE, iMIA, SIFI study groups. Predictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint: A Pooled Analysis. Spine. Published Ahead-of-Print March 27, 2017. doi:10.1097/BRS.0000000000002169
- MacBarb RF, Lindsey DP, Woods SA, Lalor PA, Gundanna MI, Yerby SA. Fortifying the Bone-Implant Interface Part II: An In Vivo Evaluation of 3D-Printed and TPS-Coated Triangular Implants. Int J Spine Surg. 2017;11. [Accepted, publication pending]
SOURCE SI-BONE, Inc.