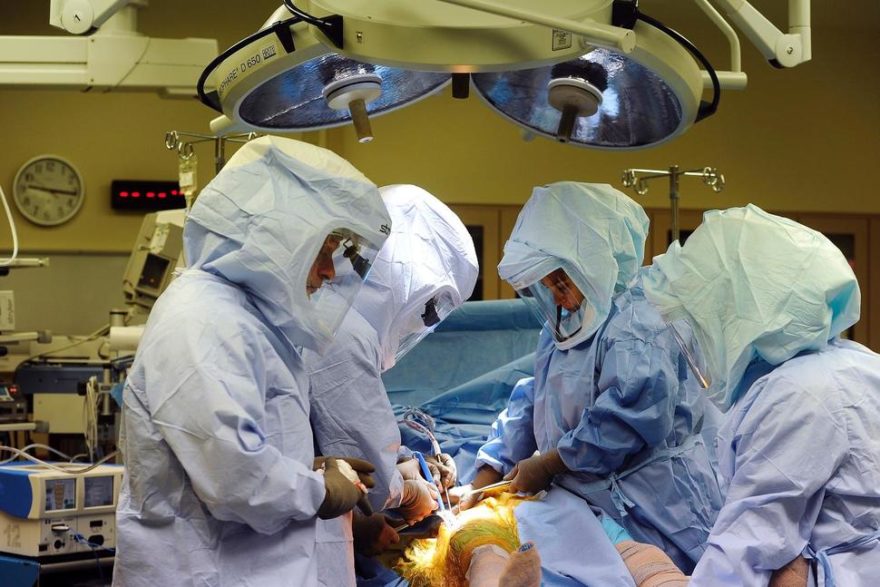
CAMBRIDGE, Mass.–(BUSINESS WIRE)–InVivo Therapeutics Holdings Corp. (NVIV) today announced that a new patient with a T8-9 fracture dislocation injury has been enrolled into The INSPIRE Study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold™ for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury) at the Carolinas Medical Center in Charlotte, NC. Domagoj Coric, M.D., of Carolina Neurosurgery and Spine Associates, Chief of Neurosurgery at the Carolinas Medical Center and a member of the INSPIRE Study Steering Committee, performed the implantation with his partner, Samuel Chewning, M.D. Surgery was performed approximately 19 hours after the injury occurred. Dr. Coric and Dr. William Bockenek, Chief Medical Officer at Carolinas Rehabilitation, are Co-principal Investigators at this site.
“Implantation of the Neuro-Spinal Scaffold went smoothly and the patient is doing well,” Dr. Coric said. “I have now implanted five patients with different injury types and locations, and the implantation procedure has been consistently uncomplicated. I look forward to following this most recent patient’s progress.”
Mark Perrin, InVivo’s Chief Executive Officer and Chairman, said, “We now have ten INSPIRE patients enrolled and in follow up, which is an important milestone in this study designed to enroll 20 evaluable patients. We thank Dr. Coric and his team for having enrolled five patients at their site, and look forward to continuing to make progress toward full enrollment.”
For more information, please visit the company’s ClinicalTrials.gov registration site: http://clinicaltrials.gov/ct2/show/study/NCT02138110
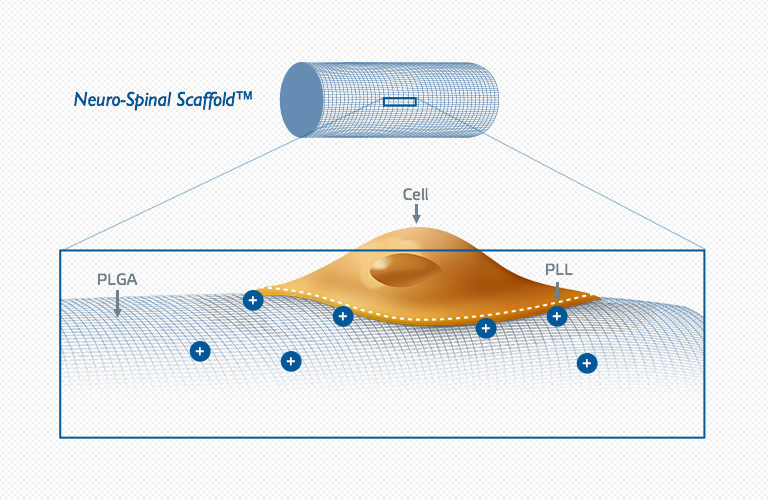
About the Neuro-Spinal Scaffold™ Implant
Following acute spinal cord injury, surgical implantation of the biodegradable Neuro-Spinal Scaffold within the decompressed and debrided injury epicenter is intended to support appositional healing, thereby reducing post-traumatic cavity formation, sparing white matter, and allowing neural regeneration across the healed wound epicenter. The Neuro-Spinal Scaffold, an investigational device, has received a Humanitarian Use Device (HUD) designation and currently is being evaluated in the INSPIRE pivotal probable benefit study for the treatment of patients with complete (AIS A) traumatic acute spinal cord injury.
About InVivo Therapeutics
InVivo Therapeutics Holdings Corp. is a research and clinical-stage biomaterials and biotechnology company with a focus on treatment of spinal cord injuries. The company was founded in 2005 with proprietary technology co-invented by Robert Langer, Sc.D., Professor at Massachusetts Institute of Technology, and Joseph P. Vacanti, M.D., who then was at Boston Children’s Hospital and who now is affiliated with Massachusetts General Hospital. In 2011, the company earned the David S. Apple Award from the American Spinal Injury Association for its outstanding contribution to spinal cord injury medicine. In 2015, the company’s investigational Neuro-Spinal Scaffoldreceived the 2015 Becker’s Healthcare Spine Device Award. The publicly-traded company is headquartered in Cambridge, MA. For more details, visit www.invivotherapeutics.com.
Safe Harbor Statement
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements within the meaning of the federal securities laws. These statements can be identified by words such as “believe,” “anticipate,” “intend,” “estimate,” “will,” “may,” “should,” “expect,” “designed to,” “potentially,” and similar expressions, and include statements regarding the safety and effectiveness of the Neuro-Spinal Scaffold and the expected timing of additional enrollment. Any forward-looking statements contained herein are based on current expectations, and are subject to a number of risks and uncertainties. Factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the company’s ability to successfully open additional clinical sites for enrollment and to enroll additional patients; the timing of the Institutional Review Board process; the company’s ability to commercialize its products; the company’s ability to develop, market and sell products based on its technology; the expected benefits and efficacy of the company’s products and technology in connection with the treatment of spinal cord injuries; the availability of substantial additional funding for the company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and other risks associated with the company’s business, research, product development, regulatory approval, marketing and distribution plans and strategies identified and described in more detail in the company’s Annual Report on Form 10-K for the year ended December 31, 2015, and its other filings with the SEC, including the company’s Form 10-Qs and current reports on Form 8-K. The company does not undertake to update these forward-looking statements.