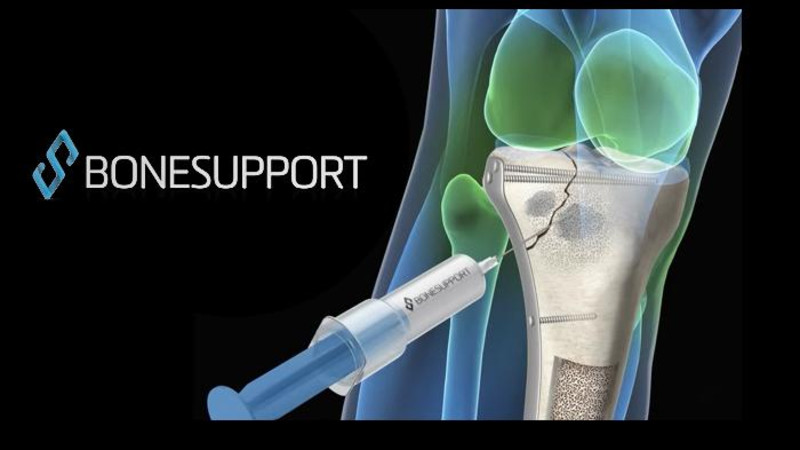
DURHAM, N.C.–(BUSINESS WIRE)–Bioventus, a global leader in orthobiologics, today announced that, effective June 1, 2017, its full surgical orthobiologics portfolio is available through Premier, Inc. Premier is a health care improvement company comprised of 3,750 US hospitals and more than 130,000 other provider organizations throughout the country. Bioventus Surgical has numerous offerings for bone healing and spine fusion including allograft bone, synthetic bone graft substitutes and cell and marrow extraction needles.
OSTEOAMP®, an allograft fusion solution, has been available through Premier since 2012. Additional products in the new agreement include: SIGNAFUSE™, a bioactive bone graft substitute, EXPONENT™ demineralized bone matrix, PUREBONE® demineralized cancellous bone and cancellous chips, INTERFACE bioactive bone graft, OSTEOMATRIX® biphasic mineral/collagen bone graft, OSTEOPLUS® biphasic mineral bone graft, CELLXTRACT®, an autologous cell and marrow extraction device, and EXTRACTOR™ autologous cell and marrow extraction device.
“The Bioventus Surgical portfolio is designed to meet the needs of surgeons and their patients, across a broad range of clinical situations, procedures, and costs,” said Henry Tung, MD, Senior Vice President, Bioventus and President, Bioventus Surgical. “We expect the Premier alliance of hospitals and healthcare providers to benefit greatly from our clinically supported and cost-effective orthobiologic solutions.”
About Bioventus
Bioventus is an orthobiologics company that delivers clinically proven, cost-effective products that help people heal quickly and safely. Its mission is to make a difference by helping patients resume and enjoy active lives. The company has two product portfolios for orthobiologics, Bioventus Active Healing Therapies and Bioventus Surgical that make it a global leader in active orthopaedic healing. Its EXOGEN® Ultrasound Bone Healing System uses safe, effective low intensity pulsed ultrasound (LIPUS) to stimulate the body’s natural healing process. EXOGEN has been used to treat more than 1 million patients worldwide and numerous regulatory agencies including the FDA, Health Canada, BSi, TGA, Medsafe, UAE Ministry of Health and SFDA have granted their approval of the product. Today it is the leading bone healing system in the market with complaints for lack of efficacy averaging less than 1%.
Built on a commitment to high quality standards, evidence-based medicine and strong ethical behavior, Bioventus is a trusted partner for physicians worldwide. For more information, visit www.BioventusGlobal.com and follow the company on Twitter @Bioventusglobal.
Bioventus, the Bioventus logo, CELLXTRACT, OSTEOAMP, OSTEOMATRIX, OSTEOPLUS, PUREBONE and EXOGEN are registered trademarks, and EXPONENT, EXTRACTOR and SIGNAFUSE are trademarks of Bioventus LLC.
Summary of Indications for Use
Please see Instructions for Use for complete lists of indications, contraindications, warnings, and precautions on the product labels, at www.BioventusSurgical.com, or by calling 1-800-637-4391.
CELLXTRACT is intended for use for aspiration of bone marrow or autologous blood using a standard piston syringe.
EXPONENT Demineralized Bone Matrix is indicated for bony voids or gaps that are not intrinsic to the stability of the bony structure. It is intended to be gently packed into bony voids or gaps of the skeletal system (posterolateral spine). These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone.
EXTRACTOR is intended for the purpose of harvesting bone marrow.
INTERFACE Bone Void Filler is indicated for bony voids or gaps that are not intrinsic to the stability of bony structures. These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone. INTERFACE Bone Void Filler is indicated to be gently packed into bony voids or gaps of the skeletal system (extremities and pelvis), and in the posterolateral spine when mixed with autograft. The product provides a bone void filler that resorbs and is replaced with bone during the healing process.
OSTEOAMP may be used in situations where an autograft is appropriate. It should be restricted to homologous use for the repair, replacement or reconstruction of musculoskeletal defects.
OSTEOMATRIX is indicated for use in bony voids or gaps that are not intrinsic to the stability of the bony structure. The product should be gently packed into bony voids or gaps of the skeletal system (i.e., the extremities, posterolateral spine and pelvis). These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone.
OSTEOPLUS is intended for use as a bone void filler for bony voids or gaps of the skeletal system (e.g., extremities, spine and pelvis) that are not intrinsic to the stability of the bony structure. OSTEOPLUS can be used with autograft as a bone graft extender. These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone.
PUREBONE Demineralized Cancellous Tissue is restricted to use by a licensed physician. PUREBONE Demineralized Cancellous Tissue can be used in orthopedic, neurosurgical and reconstructive bone grafting procedures for homologous use for the repair, replacement, or reconstruction of musculoskeletal defects. It can be used by itself as a bone graft or in conjunction with autologous bone and other forms of allograft bone. PUREBONE Demineralized Cancellous Tissue has been tested for sterility and is ready for use. Do not subject the product to additional disinfection or sterilization procedures. PUREBONE Demineralized Cancellous Tissue is intended for single patient use only. In order to prevent contamination of the graft, any open and unused PUREBONE Demineralized Cancellous Tissue must be discarded and not used in other patients.
SIGNAFUSE is a bone void filler device intended for use in bony voids or gaps that are not intrinsic to the stability of bony structure. These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone. SIGNAFUSE is indicated to be packed gently into bony voids or gaps of the skeletal system (i.e., extremities, pelvis and posterolateral spine fusion procedures). SIGNAFUSE can also be used with autograft as a bone graft extender in the posterolateral spine. The device provides a bone void filler that is resorbed and replaced with host bone during the healing process.



 About Bone Therapeutics
About Bone Therapeutics