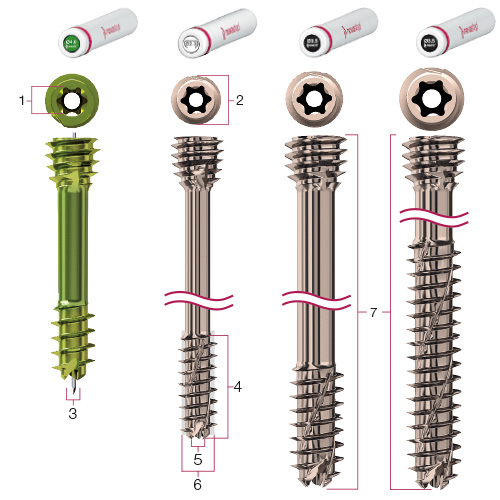
Orangeburg, New York, Oct. 07, 2016 (GLOBE NEWSWIRE) — Novastep, Inc., and its affiliates (“Novastep” or the “Company”), a global medical technologies company specializing in the foot and ankle, has further expanded its coverage of midfoot and rearfoot indications with the addition of a full range of Nexis® 5.0 and 7.0mm headless, cannulated compression screws.
The implants feature all of the hallmark design attributes inherent to the Company’s Nexis® brand, including helical thread flutes that are self-drilling, self-tapping and reverse-cutting; Torx drive recess interfaces for optimal force distribution and tapered, self-penetrating compression cones. Loïc Girod, Novastep’s Vice President of Research and Development, noted that “The Nexis® system provides surgeons with a complete and versatile selection of sterile packaged, low-profile, cannulated screws that are designed to address a broad spectrum of midfoot and hindfoot pathologies.”
The system is likewise supported with a robust instrumentation platform that is neatly stored in a versatile, light-weight, space-saving tray to combine the Nexis® 5.0 and 7.0mm instruments with interchangeable Nexis® 4.0mm headless screw or Arcad 18/20/25mm nitinol compression clip instrument modules that serve to further enhance its versatility. “Operating room efficiency and cost reduction are prime areas of focus for Novastep” said Joseph Larsen, DPM of ProHealthCare Foot & Ankle in New York; adding that “Novastep’s implant and cleanSTART® deployment technologies provide a systematized logistics platform that is easily customizable to fit virtually anyone’s needs and substantially reduces inventory requirements and sterile field volumes.”
All Novastep implants are packaged sterile in quickSTACK™ containers or quickTUBE™ nested cylinders, depending on the size and configuration of the product. The STACKS and TUBES are housed and organized in the cleanSTART® Implant Dispenser console, which may be uniquely tailored to address individual surgeon preferences.
Nexis® and other key elements of Novastep’s portfolio will be featured at the Desert Foot 2016 meeting on October 19–22 in Phoenix, AZ and the upcoming 2016 Global Foot and Ankle Symposium in New York City on December 2–3.
About Novastep
Novastep is a global medical device company specializing in the design, development and commercialization of advanced technologies that treat conditions affecting the foot and ankle. The Company is focused on optimizing clinical efficiencies, inventory management and healthcare economics by transforming the way foot and ankle products are deployed and utilized in the surgical environment. Novastep has allied itself with a strategic network of key international opinion leaders to deliver breakthrough technologies, innovative services and compelling medical education programs to the foot and ankle community. Novastep’s portfolio, services and distribution platforms are uniquely positioned to address foot and ankle trauma, deformity corrections and Charcot fracture management.
For further information concerning this announcement and/or Novastep, Inc., send all inquiries to info@novasteportho.com or call 877.287.0795.
Related Links
For general information visit: novastep-us.com