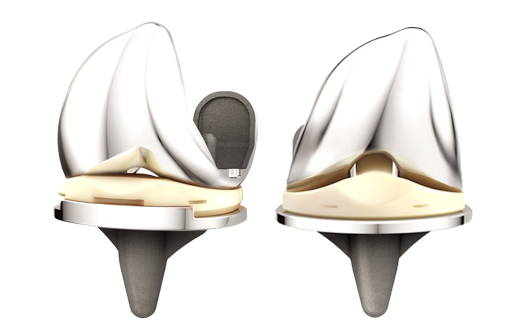
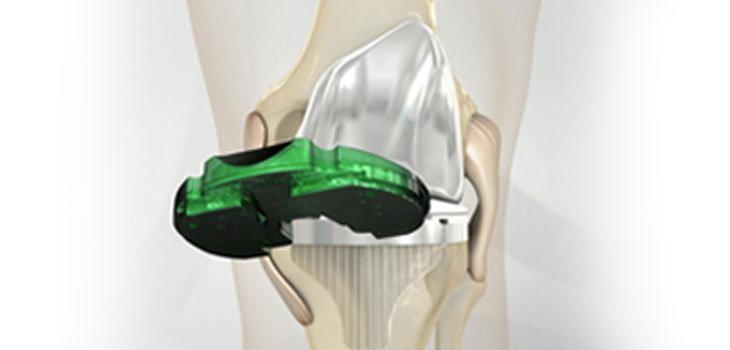
SAN DIEGO, March 14, 2017 /PRNewswire/ — Today, DePuy Synthes*, part of the Johnson & Johnson Family of Companies, released a new report, “Improving the Value of Primary Total Knee Arthroplasty: the ATTUNE® Knee System,” that analyzes the ATTUNE Knee’s current clinical and economic data. The report concludes that in a value-based healthcare environment with cost constraints and growing procedure volumes, the value of a knee replacement implant is measured not only by how long the implant lasts (implant survivorship), but also through patient reported outcomes and the economic benefits of the procedure.
The report, which was released alongside the American Academy of Orthopaedic Surgeons Annual Meeting in San Diego, is authored by David Fisher**, M.D., Director of the Total Joint Center of Excellence at OrthoIndy Hospital in Indianapolis, Indiana, and Professor David Parkin, an Honorary Visiting Professor at City, University of London and a Senior Visiting Fellow, Office of Health Economics.
The report analyzes approaches for evaluating primary total knee arthroplasty (TKA) from a clinical and health economic perspective, and assesses the current data from DePuy Synthes’ ATTUNE Knee Evidence Generation Program. This program, the largest of its kind in DePuy Synthes’ history, includes a wide range of data from company-initiated studies, investigator-initiated studies, independent studies and national joint registries.
In their analysis, Dr. Fisher and Professor Parkin conclude that, “Based on available data, the ATTUNE Knee appears to be advancing outcomes for patients and creating value for clinicians, providers and payors in a challenging and dynamic healthcare environment.”
Click to Tweet: New @DePuySynthes report released today demonstrates the clinical and economic value of the ATTUNE® Knee System #AAOS17 http://bit.ly/2mjUTL8
This report comes at a time when the entire episode of patient care is a major focus for global healthcare providers, placing a laser-like focus on both improving patient outcomes and managing costs. The bundled payments program in the United States and the “Payments for Results” system in England are two examples of the drive to minimize the total cost of care while maintaining quality. Against this backdrop, data from a comprehensive evidence generation program can help provide evidence to support decisions about a device’s overall value.
“The success of any knee replacement is multifactorial, and the data on the ATTUNE Knee gives me confidence that I’m using a knee replacement that is delivering value for patients and the healthcare system,” said Dr. Fisher.
Added Professor Parkin: “Quality of life, as measured by patient reported outcomes, has been shown to be a driver of cost effectiveness in knee replacement. The evidence that I’ve seen about the effectiveness of the ATTUNE Knee in improving patient reported outcomes suggests it can deliver a better quality of life for patients compared to other leading knee systems. Therefore, the ATTUNE Knee may potentially play a role in helping reduce some of the healthcare and societal costs associated with knee osteoarthritis.”
According to the analysis, the ATTUNE Knee has thus far demonstrated favorable survivorship, improved patient reported outcomes versus other knee systems, as well as potential economic benefits:
- Survivorship: The published report from the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man (NJR) showed that the ATTUNE Knee’s estimated cumulative percent revision was 1.39% at three years (98.61% survivorship) for 4,463 knees, comparing favorably to the class of Cemented Total Knee Arthroplasty that has an estimated cumulative percent revision of 1.50%1. In addition, per the 2016 Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), in which 4,831 ATTUNE Knees are being tracked, the ATTUNE Knee’s estimated cumulative percent revision was 0.5% (ATTUNE Cruciate Retaining Knee) and 0.4% (ATTUNE Posterior Stabilized Knee) at one year.2 This compares favorably to the overall class of cemented TKA at one year, which has an estimated cumulative percent revision of 1.0 percent.2
- Patient Reported Outcomes: DePuy Synthes has taken the lead in this area by helping generate and validate a new measure, the Patient’s Knee Implant Performance (PKIP), to specifically assess the functional status of a patient’s knee from their own perspective. Results of a study using PKIP as well as other commonly used generic and condition-specific patient reported outcome measures showed that, at one year, study participants implanted with the ATTUNE Knee had statistically significant improvements compared to other leading knee systems in terms of confidence in stability during activity, decreased anterior knee pain, activities of daily living and quality of life.3
- Health Economics: A U.S. hospital database analysis showed 39% lower odds of patient discharge to a skilled nursing facility when implanted with an ATTUNE Knee, compared to patients who received total knee replacement with a Triathlon® Knee.4 This may potentially impact patient satisfaction and reduce healthcare costs. Post discharge costs are a significant portion of the overall episode of care. One study showed that up to 35% of episodic costs were related to post hospital discharge care5 and another showed it was up to 50%.6
To read this report and learn more about ATTUNE Knee Evidence, visit www.ATTUNEevidence.com.
About DePuy Synthes Companies
DePuy Synthes Companies, part of the Johnson & Johnson Family of Companies, provides one of the most comprehensive orthopaedics portfolios in the world. DePuy Synthes solutions, in specialties including joint reconstruction, trauma, craniomaxillofacial, spinal surgery and sports medicine, are designed to advance patient care while delivering clinical and economic value to health care systems worldwide. For more information, visit www.depuysynthes.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the effectiveness and value of the ATTUNE Knee System. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of DePuy Synthes and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; product efficacy or safety concerns resulting in product recalls or regulatory action; manufacturing difficulties and delays; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns for health care products and services; and trends toward health care cost containment. A further list and description of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended January 1, 2017, including in the sections captioned “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements”, and the company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither DePuy Synthes nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
*DePuy Synthes represents the products and services of DePuy Synthes, Inc. and its affiliates.
**Consultant to DePuy Synthes Companies
The third party trademarks used herein are the trademarks of their respective owners.
DSUS/JRC/0217/2031 March 2017
References:
|
1 |
National Joint Registry for England, Wales, Northern Ireland and the Isle of Man, 13th Annual Report. (2016). Table 3.24 |
|
(a). Available from: www.njrreports.org.uk |
|
|
2 |
Australian Orthopaedic Association National Joint Replacement Registry Annual Report. (2016). Tables KT9 and KT22. Retrieved from: |