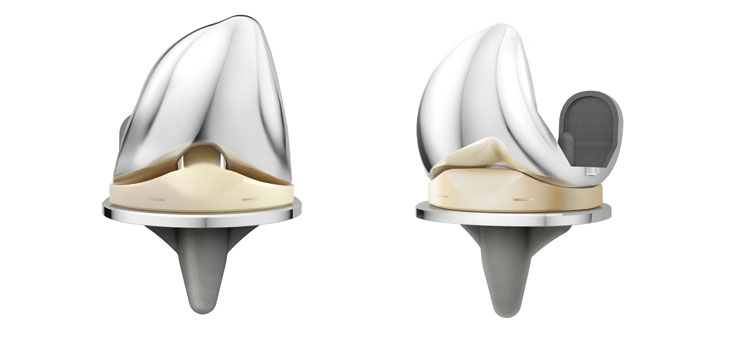
RAYNHAM, Mass., Dec. 12, 2016 /PRNewswire/ — OMNIlife science, Inc. (“OMNI™”), an established medical technology company targeting the rapidly growing $15 billion global hip and knee replacement medical device sector, today announced the tenth anniversary of the launch of their APEX Knee™ System. Since 2006, the APEX Knee has been used in more than 75,000 total knee replacement procedures worldwide.
Ten years is a significant milestone in the lifetime of orthopedic implants because the results at ten years can generally be used to extrapolate the long term survivorship of the implant. Any device or design-related issues should have become apparent by this time, so device failures would likely be due to wear of the polyethylene components. This is not an issue for the APEX Knee polyethylene because it is EtO sterilized and it has more than 30 years of proven success in other medical devices. According to the latest Australian Registry statistics, the APEX Knee has a survivorship of 99.5%, better than any of its competitors. *
“Our knee implant design philosophy is that the device should allow the patient’s knee kinematics to be dominated by their individual anatomy, not by the implant; that the polyethylene should never see ionizing radiation, and that the instruments should be simple and easy to use,” said George Cipolletti, OMNI co-founder and Chief Technology Officer. “I am really proud that we have accomplished our goal: happy surgeons with happy patients!”
OMNI worked closely with two physicians, Warren Low**, M.D. and Thomas Tkach**, M.D., both at McBride Orthopedic Hospital in Oklahoma City, Oklahoma, to design the APEX Knee. As the first surgeons to implant the knee, they are pleased with its success. “I think the APEX Knee is an excellent implant in terms of sizing, fit, and kinematics as well as in instrumentation,” said Dr. Low. “The APEX Knee has stood the test of time and surgeons are using it in increasing numbers, so they and their patients are demonstrating its efficacy.”
Dr. Tkach commented, “When we started the APEX Knee project with OMNI 10 years ago our goal was simple. To take the experience of the design team accumulated over many years in the OR and in the orthopaedic industry and design a ‘no compromise’ completely new total knee replacement system. I believe we achieved our goal and both my personal clinical experience, as well as that reported from surgeons around the world, indicate that we have created a world class product that remains state of the art in design and performance 10 years after its first implantation. I look forward to seeing what the next 10 years will bring as we continue to improve the implants and instruments that make the APEX Knee System work so well in the hands of so many different surgeons in so many different countries.”
In addition to their hip and knee implants, OMNI has developed OMNIBotics®, an innovative technology platform for robotic-assisted total knee replacement and computer-assisted total hip replacement, enabling a customized procedure for each patient. OMNIBotics is the leading robotic- assisted total knee replacement technology available, with more than 10,000 OMNIBotics procedures performed worldwide.
“The APEX Knee is the foundation of OMNI,” said Rick Randall, CEO. “We have a five year history of combining this outstanding and proven implant with our OMNIBotics technology, resulting in a procedure that goes beyond traditional total knee replacement surgery. It is designed to enable rapid patient recovery through improved implant alignment and kinematic function. We believe it is transformational in its precision and patient benefits.”
About OMNI
OMNI is a privately held company with a proprietary robotic platform, OMNIBotics, which allows surgeons to conduct patient-specific total knee surgery designed to enhance patient satisfaction and reduce hospital costs. In addition, OMNI designs, engineers, manufactures and distributes a wide range of proprietary hip and knee implants and is focused on providing cutting edge technologies to transform outcomes in joint replacement surgery and improve patient care. For more information about OMNI, please visit www.omnils.com.
Forward-Looking Statements
Statements in this press release concerning the future business, operations and prospects of OMNIlife science, Inc., including statements using the terms “plans,” “believes” or similar expressions are “forward- looking” statements as defined in the Private Securities Litigation Reform Act of 1995. These statements are based upon management’s current expectations and are subject to a number of factors and uncertainties. Information contained in these forward-looking statements is inherently uncertain, and actual performance and results may differ materially due to many important factors. Such factors include, among others, changes in competitive conditions and pricing in OMNI’s markets, decrease in the demand for OMNI’s products, delays in OMNI’s product research and development cycles, decreases in the use of OMNI’s principal product lines or in procedure volume, unanticipated issues in complying with domestic or foreign regulatory requirements related to OMNI’s current products or securing regulatory clearance or approvals for new products or upgrades or changes to OMNI’s current products, the impact of the United States healthcare reform legislation on hospital spending and reimbursement, any unanticipated impact arising out of the securities class action or any other litigation, inquiry, or investigation brought against OMNI, increases in costs of OMNI’s sales force and distributors, and unanticipated intellectual property expenditures required to develop, market, and defend OMNI’s products. OMNI cannot guarantee any future results, levels of activity, performance or achievement. OMNI undertakes no obligation to update any of its forward-looking statements after the date of this press release.
Contact
Cindy Holloway, Director of Marketing Communications
Phone: (508) 824-2444
*Australian Orthopaedic Association National Joint Replacement Registry. Annual Report. Adelaide: AOA; 2016
**Both Warren Low and Thomas Tkach have product development agreements with OMNIlife science, Inc.
SOURCE OMNIlife science, Inc.