BELGRADE, Mont., Nov. 23, 2016 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE MKT:XTNT), a leader in the development of regenerative medicine products and medical devices, today announced that the U.S. Food and Drug Administration (FDA) has cleared the Xsert Lumbar Expandable Interbody System.
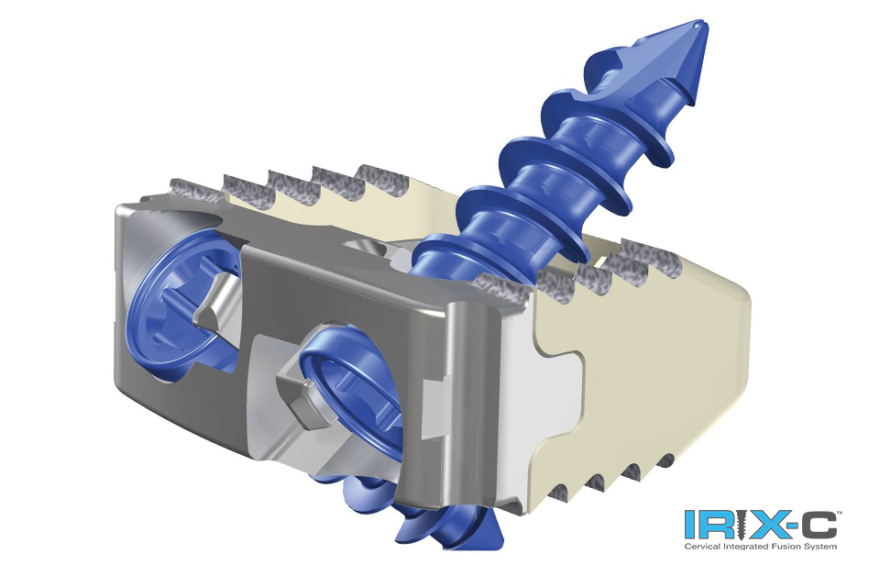
The Xsert System is an all titanium expandable interbody device that expands in-situ. It is available in various sizes and lordotic angulations to fit the most complex anatomical needs of patients. These spacers are designed to simplify surgeon insertion technique[s] while offering implant height adjustability. Osteointegration will be encouraged through nanotextured endplate surfaces as well as central and lateral implant graft windows. The system is intended for use at one or two contiguous levels (L2-S1 inclusive).
Xsert is also cleared to be packed with autograft or allograft bone graft. Xtant Medical’s 3Demin and patented OsteoSponge technology are excellent allografts to use with Xsert due to their ability to compress, fill and expand in the device’s graft chamber, allowing for ideal bone contact and fusion.
“We are very pleased to have received clearance for Xsert. This system allows the surgeon to implant the cage in a tighter corridor and then expand the device according to the patient’s anatomy,” stated Dr. David Kirschman, developer of Xsert and current member of the Xtant Medical Board of Directors. “With the clearance of allograft use with Xsert, Xtant Medical further expands combined device and biologic solutions for surgeons and their patients.”
Xtant Medical estimates the U.S market for lumbar interbody devices at $1.3M and growing. The worldwide market for Demineralized Bone Matrix (DBM) is estimated at $485M. Xsert will be available in an initial product release in mid 2017.
About Xtant Medical Holdings
Xtant Medical develops, manufactures and markets regenerative medicine products and medical devices for domestic and international markets. Xtant Medical products serve the specialized needs of orthopedic and neurological surgeons, including orthobiologics for the promotion of bone healing, implants and instrumentation for the treatment of spinal disease, tissue grafts for the treatment of orthopedic disorders, and biologics to promote healing following cranial, and foot and ankle surgeries. With core competencies in both biologic and non-biologic surgical technologies, Xtant Medical can leverage its resources to successfully compete in global neurological and orthopedic surgery markets. For further information, please visit www.xtantmedical.com.
Important Cautions Regarding Forward-looking Statements
This press release contains certain disclosures that may be deemed forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to significant risks and uncertainties. Forward-looking statements include statements that are predictive in nature, that depend upon or refer to future events or conditions, or that include words such as “continue,” “efforts,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” “projects,” “forecasts,” “strategy,” “will,” “goal,” “target,” “prospects,” “potential,” “optimistic,” “confident,” “likely,” “probable” or similar expressions or the negative thereof. Statements of historical fact also may be deemed to be forward-looking statements. We caution that these statements by their nature involve risks and uncertainties, and actual results may differ materially depending on a variety of important factors, including, among others: our ability to integrate the acquisition of X-spine Systems, Inc. and any other business combinations or acquisitions successfully; our ability to remain listed on the NYSE MKT; our ability to obtain financing on reasonable terms; our ability to increase revenue; our ability to comply with the covenants in our credit facility; our ability to maintain sufficient liquidity to fund our operations; the ability of our sales force to achieve expected results; our ability to remain competitive; government regulations; our ability to innovate and develop new products; our ability to obtain donor cadavers for our products; our ability to engage and retain qualified technical personnel and members of our management team; the availability of our facilities; government and third-party coverage and reimbursement for our products; our ability to obtain regulatory approvals; our ability to successfully integrate recent and future business combinations or acquisitions; our ability to use our net operating loss carry-forwards to offset future taxable income; our ability to deduct all or a portion of the interest payments on the notes for U.S. federal income tax purposes; our ability to service our debt; product liability claims and other litigation to which we may be subjected; product recalls and defects; timing and results of clinical studies; our ability to obtain and protect our intellectual property and proprietary rights; infringement and ownership of intellectual property; our ability to remain accredited with the American Association of Tissue Banks; influence by our management; our ability to pay dividends; our ability to issue preferred stock; and other factors.
Additional risk factors are listed in the Company’s Annual Report on Form 10-K and Quarterly Reports on Form 10-Q under the heading “Risk Factors.” You should carefully consider the trends, risks and uncertainties described in this document, the Form 10-K and other reports filed with or furnished to the SEC before making any investment decision with respect to our securities. If any of these trends, risks or uncertainties actually occurs or continues, our business, financial condition or operating results could be materially adversely affected, the trading prices of our securities could decline, and you could lose all or part of your investment. The Company undertakes no obligation to release publicly any revisions to any forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law. All forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by this cautionary statement.
Investor Contact CG CAPITAL Rich Cockrell 877.889.1972 xtant@cg.capital Company Contact Xtant Medical Molly Mason mmason@xtantmedical.com