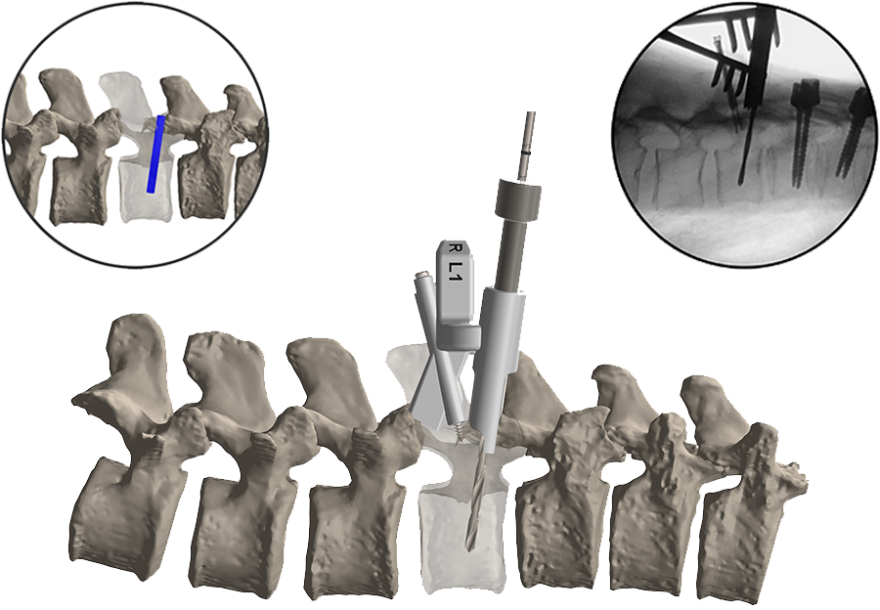
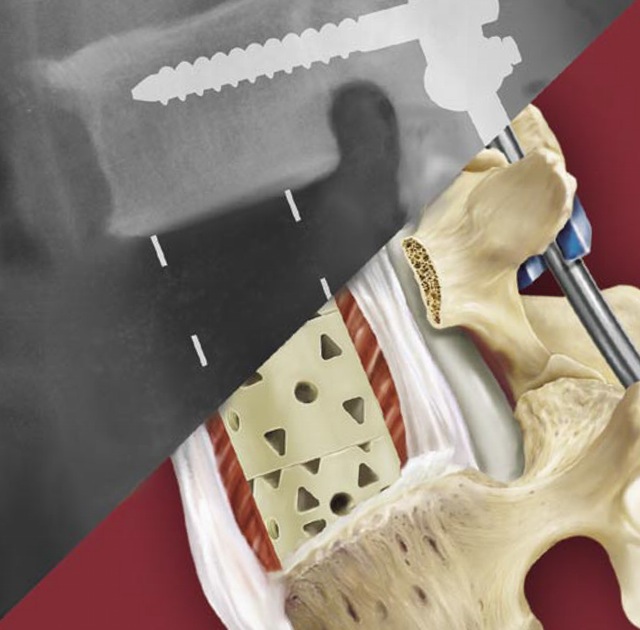
CARLSBAD, Calif., Nov. 07, 2016 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation(NASDAQ:SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Mariner™ Posterior Fixation System.
The Mariner Posterior Fixation System is a pedicle screw system featuring modular threaded technology and accompanying instrumentation. Designed to reduce the number of trays needed for surgery, Mariner is intended to provide surgeons with multiple intra-operative options to facilitate posterior lumbar fixation. Key market differentiators of the Mariner System include in-situ modularity, motion limiting heads and rod versatility with both 5.5mm and 6.0mm offerings.
“In today’s hospital environment, the key is to have as many options as you can for your patient without overburdening your staff,” stated Douglas Orndorff, MD. “Mariner is versatile, yet simple to use. Ultimately, it lets me make intraoperative decisions seamlessly.”
Dr. Warren Yu, Director of Spine Surgery at George Washington Hospital commented, “Mariner’s state-of-the-art instrumentation and modular screw design provide me with the broad selection of implant configurations that I need to address the challenging patient anatomy I see in my adult spine practice – from basic degenerative to complex deformity cases.”
SeaSpine is conducting initial cases with the Mariner Posterior Fixation System through a limited launch that will continue over the coming months. The Company expects a full commercial launch of the Mariner System in the first half of 2017.
“The Mariner Poster Fixation System brings innovative features to the market and utilizes a modular design that increases surgeon flexibility while reducing the number of trays that need to be brought into the operating room,” stated Keith Valentine, Chief Executive Officer of SeaSpine. “The Mariner launch is an important enhancement to SeaSpine’s spinal hardware product offerings in the $1.8 billion posterior lumbar fixation market.”
About SeaSpine
SeaSpine (www.seaspine.com) is a global medical technology company focused on the design, development and commercialization of surgical solutions for the treatment of patients suffering from spinal disorders. SeaSpine has a comprehensive portfolio of orthobiologics and spinal hardware solutions to meet the varying combinations of products that neurosurgeons and orthopedic spine surgeons need to perform fusion procedures on the lumbar, thoracic and cervical spine. SeaSpine’s orthobiologics products consist of a broad range of advanced and traditional bone graft substitutes that are designed to improve bone fusion rates following a wide range of orthopedic surgeries, including spine, hip, and extremities procedures. SeaSpine’s spinal hardware portfolio consists of an extensive line of products to facilitate spinal fusion in minimally invasive surgery (MIS), complex spine, deformity and degenerative procedures. Expertise in both orthobiologic sciences and spinal fusion hardware product development helps SeaSpine to offer its surgeon customers a complete solution to meet their fusion requirements. SeaSpine currently markets its products in the United States and in over 30 countries worldwide.
Forward-Looking Statements
SeaSpine cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that are based on the Company’s current expectations and assumptions. Such forward-looking statements include, but are not limited to, statements relating to: the design benefits of the Mariner Posterior Fixation System, including its potential to reduce the number of trays needed for surgery and to provide surgeons with multiple intra-operative options to facilitate posterior lumbar fixation; and the timing and success of both the limited and full commercial launch of the Mariner Posterior Fixation system. Among the factors that could cause or contribute to material differences between our actual results and the expectations indicated by our forward-looking statements are risks and uncertainties that include, but are not limited to: the fact that the Marine Posterior Fixation System has not been validated clinically and may require substantial additional development activities, which could introduce unexpected expense and delay, including potentially requiring resubmission of one or more products to FDA for clearance, which clearance cannot be certain, whether on a timely basis or at all; surgeons’ willingness to use the Mariner Posterior Fixation System; the risk that the Mariner Posterior Fixation System may not demonstrate adequate safety or efficacy, independently or relative to competitive products, to support a full commercial launch; the risk of supply shortages, including as a result of our dependence on a limited number of third-party suppliers for components and raw materials, or otherwise; and other risks and uncertainties more fully described in our news releases and periodic filings with the Securities and Exchange Commission. The Company’s public filings with the Securities and Exchange Commission are available at www.sec.gov.
You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date when made. SeaSpine does not intend to revise or update any forward-looking statement set forth in this news release to reflect events or circumstances arising after the date hereof, except as may be required by law.
Investor Relations Contact
Lynn Pieper
(415) 937-5402
ir@seaspine.com