WARSAW, Ind., March 14, 2017 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global leader in musculoskeletal healthcare, today announced plans to highlight its latest commercial offerings and preview its next generation of technological innovations at the American Academy of Orthopaedic Surgeons (AAOS) annual meeting this week in San Diego, CA. The Company’s state-of-the-art booth (#4333) will feature an immersive and interactive tour of its newly launched digital technology and clinical services offering, Zimmer Biomet Signature Solutions, alongside more than 50 new products from its vast and diversified portfolio.
“We’re proud and excited to showcase our most innovative new commercial offerings to the largest gathering of orthopaedic professionals,” said David Dvorak, President and CEO of Zimmer Biomet. “New this year is a virtual booth experience designed to bring Zimmer Biomet Signature Solutions to life through a guided tour, a sneak preview of our emerging robotics platform and other technologies in our deep pipeline.”
Following is a snapshot of the key highlights being featured at the Zimmer Biomet booth (#4333) at AAOS 2017:
- Zimmer Biomet Signature Solutions
- Guided tours through the virtual experience every 15 minutes. Members of the press can reserve a spot by contacting Monica Kendrick at monica.kendrick@zimmerbiomet.com.
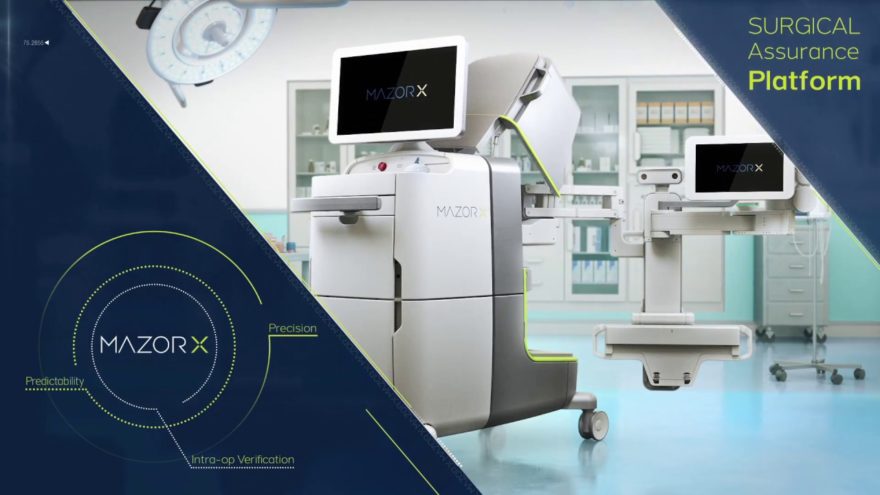
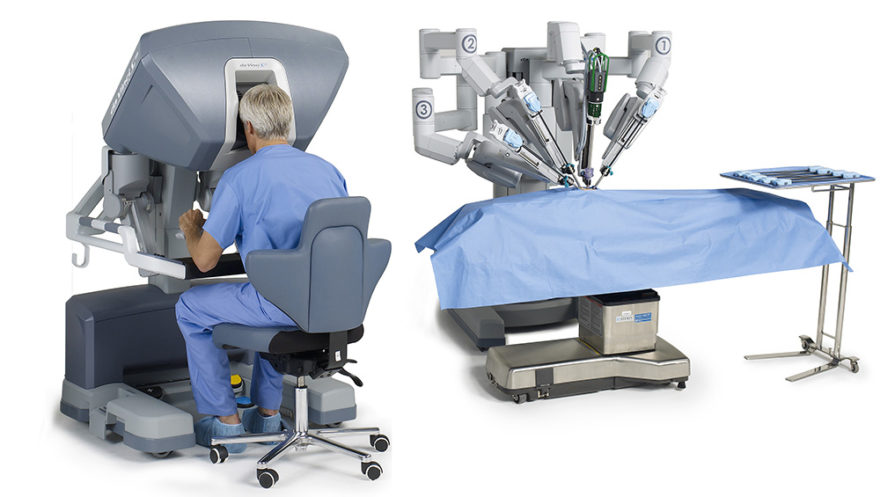
- Robotic Technology
- Catch a glimpse of the future of personalization and intelligent instrument technology through innovative robotics.i
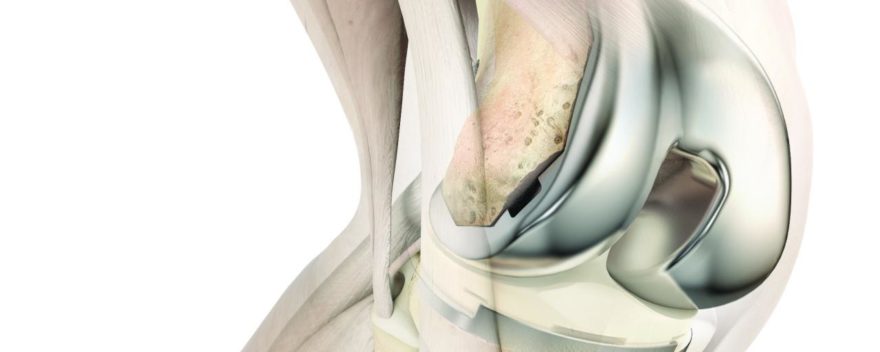
- Knees
- Vanguard® Individualized Design (ID), advances soft tissue preservation and balancing through independent medial and lateral articular surface constraint and thickness options.
- OSSTM Orthopedic Salvage System, a comprehensive modular platform providing surgeons with intraoperative flexibility often required during limb salvage procedures.
- Persona® Medial Congruent® Bearing, designed to recreate more natural feeling motion of the human knee by maximizing knee joint stability while allowing mobility.
- Hips
- Hip Preservation portfolio of options designed to treat conditions leading up to osteoarthritis and potentially preventing the need for total hip replacement.
- Trabecular MetalTM acetabular cups, which National Joint Registry analysis has recently shown are 21 percent less likely to be re-revised due to infection (statistically significant, p-value=0.036).ii
- The latest in dual mobility, Microplasty® and revision implant options designed to address the distinct needs of individual patients while simplifying surgical workflow.
- Personalized Solutions
- A comprehensive technology-based portfolio of guides, tools and software to support surgical planning, intraoperative guidance and optimal component placement.
- Bone Cement
- StageOneTM and StageOne Select Spacer Molds, designed to mold a temporary knee, hip and shoulder spacer for patients undergoing a two stage revision due to an infected total joint.
- Diagnostics
- Synovasure® Laboratory Panel for the detection of Periprosthetic Joint Infection (PJI), now includes Microbial ID. These diagnostic tests provide a fast and easy method to diagnose PJI based on the flagship test, which determines the concentration of Alpha Defensin in synovial fluid. Microbial ID detects the presence of microbes in the synovial fluid in a matter of hours.
- Extremities
- Vault Reconstruction System (VRS), the first commercially available patient-matched glenoid implant, cleared to specifically treat patients with severe glenoid bone loss and a deficient rotator cuff.
- L2LTM Radial Head System, a simple, smooth design solution for replacing the proximal radial head in patients with fractures.
- Foot and Ankle
- Deformity correcting products available through our partnership with Nextremity Solutions, Inc., including the Nextra®Hammertoe Correction System, the MSP™ Metatarsal Shortening System and the Re+Line® Bunion Correction System.
- Subchondroplasty® Procedure for foot and ankle surgery, a minimally invasive outpatient intervention that addresses the defects associated with subchondral bone marrow lesions.
- Trauma
- A.L.P.S.® Proximal Humerus Plating System, designed to minimize the risk of complications commonly associated with proximal humerus fractures, such as varus collapse, articular screw penetration and subacromial impingement.
- N-Force Fixation System® with N-Force Blue, an augmented fixation system, integrating fenestrated screws and a Bone Substitute Material into a single construct to provide improved metaphyseal void fill and increase structural support of the implant.
- RibFix Blu® Thoracic Fixation System, designed and used for the stabilization and rigid fixation of fractures in the chest wall, including sternal reconstructive surgical procedures, trauma or planned osteotomies.
- Sports Medicine
- Quattro® Link, a knotless anchor that brings control and efficiency to soft tissue repair.
- BioWickTM SureLock®, a rotator cuff implant that is an interpositional bioresorbable scaffold wick.
- SpeedSnareTM Surgical Suture Passer, which allows the ability to pass a suture through single or multiple ports.
- Gel-One® Cross-Linked Hyaluronate, the first low-volume viscosupplement available in a single-injection formula, for the treatment of pain in osteoarthritis of the knee that does not respond adequately to other conservative treatments.
- Biologics
- nSTRIDE® Autologous Protein Solution (ex-US use only), a single-shot autologous anti-inflammatory for treatment of knee osteoarthritis.
- AmnioFloTM, allograft derived from human amniotic fluid, for joint cushioning and lubrication.
- Surgical
- The IntelliCart™ System Duo Fluid Carts, the foundation for infectious waste technology with market-leading 34-liter capacity, extra quiet vacuum pump, clog-free suction manifolds and portable smoke evacuation.
- Bactisure™ Wound Lavage, clear, low-odor, aqueous solution designed to remove structurally resistant forms of bacteria, including biofilms, on all wound types.
- The VasoPress® System, reduces the risk of blood clots associated with deep vein thrombosis by propelling the venous blood out of the deep veins while patients are undergoing surgical procedures or immobile for an extended period of time.
- Spine
- Mobi-C® Cervical Disc, the first cervical disc replacement device approved for both one and two-level procedures.
- Vitality® Spinal Fixation System, an adaptable system designed for spinal fixation in complex thoracolumbar procedures.
About Zimmer Biomet
Founded in 1927 and headquartered in Warsaw, Indiana, Zimmer Biomet is a global leader in musculoskeletal healthcare. We design, manufacture and market orthopaedic reconstructive products; sports medicine, biologics, extremities and trauma products; office based technologies; spine, craniomaxillofacial and thoracic products; dental implants; and related surgical products.
We collaborate with healthcare professionals around the globe to advance the pace of innovation. Our products and solutions help treat patients suffering from disorders of, or injuries to, bones, joints or supporting soft tissues. Together with healthcare professionals, we help millions of people live better lives.
We have operations in more than 25 countries around the world and sell products in more than 100 countries. For more information, visit www.zimmerbiomet.com or follow Zimmer Biomet on Twitter at www.twitter.com/zimmerbiomet.
Cautionary Statement Regarding Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning Zimmer Biomet’s expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties that could cause actual outcomes and results to differ materially. For a list and description of some of such risks and uncertainties, see our periodic reports filed with the SEC. These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in Zimmer Biomet’s filings with the SEC. We disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be set forth in our periodic reports. Accordingly, such forward-looking statements speak only as of the date made. Readers of this news release are cautioned not to place undue reliance on these forward-looking statements, since, while management believes the assumptions on which the forward-looking statements are based are reasonable, there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this news release.
i Concept device – not for sale in the US
ii 1. According to NJR data from 2003 to 2015 where 9,573 Trabecular Metal and 30,452 non-Trabecular Metal cups were used in revision THA and based on hazard ratios adjusted by patient gender, age group, and indications (OA/non-OA).
2. NJR data shows a higher percentage of TM cups were used with antibiotic bone cement compared to all other non-TM cementless cups.
SOURCE Zimmer Biomet Holdings, Inc.