FLOWOOD, Miss., Aug. 14, 2018 /PRNewswire/ — Zavation, an employee-owned medical device company that designs, develops, manufactures and distributes medical device products, announced today the launch of a fenestrated Facet Screw system and a Sacroiliac (SI) Screw system.
FLOWOOD, Miss., Aug. 14, 2018 /PRNewswire/ — Zavation, an employee-owned medical device company that designs, develops, manufactures and distributes medical device products, announced today the launch of a fenestrated Facet Screw system and a Sacroiliac (SI) Screw system.
For more information on Zavation’s complete product portfolio, visit http://zavation.com/.
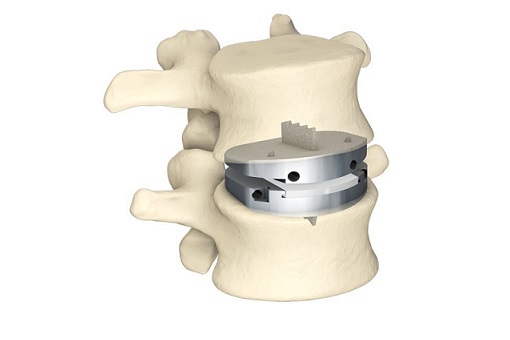
The Zavation Facet Screw system is a permanent implant device made from a cobalt chrome alloy that is implanted surgically from the posterior approach. The potentially minimally invasive system serves as a fusion and fixation system- all in one. The device is intended to provide mechanical support and stability to the implanted level until biologic fusion is achieved. Static and dynamic testing demonstrated that the Zavation Facet system is two times stronger than the predicate.
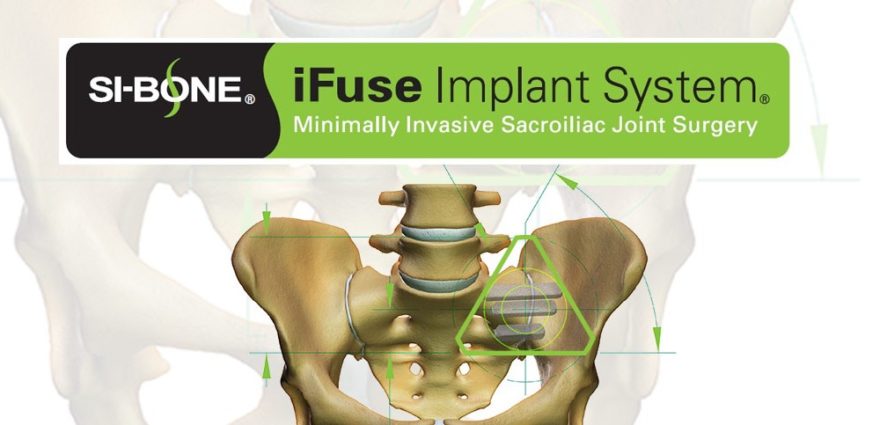
The Zavation SI System is designed to be a complete fixation system to stabilize the sacroiliac joint. The system is manufactured from a titanium alloy, the SI screw has an aggressive tip and cutting flute allowing for easy insertion. The multiple lengths (from 25-50mm in 5 mm increments), cannulated and with smooth shank options (from 30-50mm in 5 mm increments) accommodate variations in patient anatomy. The system also incorporates bone growth windows along the screw’s body which permits bone growth along the screw. The system is specifically designed with a low-profile screw head which may prevent soft tissue irritation.
Jeffrey Johnson, Zavation CEO, stated, “We are excited about receiving 510K approvals for the Facet Screw System and the SI Joint Screw System. The facet screw serves as a fusion and fixation system all-in-one, it makes for an efficient procedure. Both the Facet Screw System and SI Joint Screw System allows for surgeon preference which optimizes flow and functionality in the OR.”
Zavation will showcase the Facet Screw System and the SI Screw System at the North American Spine Society (NASS) in Los Angeles, CA September 26-29, 2018.
About Zavation Medical Products, LLC– Based in Flowood, MS, Zavation designs, engineers, and manufactures a portfolio of spinal hardware covering key areas including thoracolumbar, cervical, interbody fusion, and minimally invasive surgery.
SOURCE Zavation
Photo: Facet Screw (PRNewsfoto/Zavation) Sacroiliac Screw (PRNewsfoto/Zavation)