SAN DIEGO, July 10, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, today announced the Company will officially unveil its XLIF® Lordotic Expandable (XLX) Interbody System, which just received 510(k) clearance from the U.S. Food and Drug Administration (FDA), at the 25th International Meeting on Advanced Spine Techniques (IMAST) in Los Angeles, July 11-14, 2018. NuVasive is a Double Diamond Sponsor of the conference, reflecting its continued support of advancing spine care.
Through controlled lordotic expansion and multifunctional instrumentation, the XLIF anterior column realignment (ACR) technique offers an efficient and minimally disruptive alternative to traditional open procedures. Leveraging the clinical advantages of the XLIF ACR procedure, the XLX ACR system allows the surgeon to address sagittal alignment from the anterior column while reducing blood loss and hospital stays. Scheduled to launch in July 2018, the XLX implant will be offered in a comprehensive range of lengths and heights with up to 30° of customizable lordosis to address varying patient anatomy.
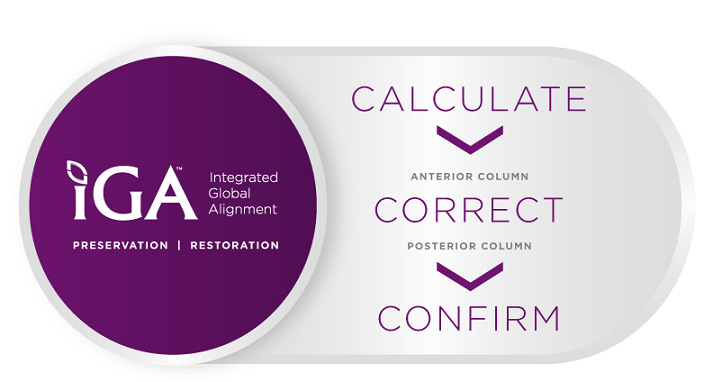
Leveraging Surgical Intelligence™, the Company’s ecosystem for better surgery, XLX ACR has the ability to improve ACR procedures overall. The Integrated Global Alignment (iGA®) software can calculate alignment parameters with the preoperative planning tools NuvaLine® and NuvaMap®, and can intraoperatively evaluate correction using real-time intraoperative assessment with NuvaMap O.R. software. Surgeons can then confirm the restoration and preservation of global sagittal alignment postoperatively.
“The XLX ACR implant adds the first expandable implant to the portfolio of pathology-specific XLIF interbody options,” said Dr. Ivan Cheng, spine surgeon and Associate Professor of Orthopedic Surgery and, by courtesy, Associate Professor of Neurosurgery at Stanford University. “We are now better able to achieve correction of alignment and save patients significant morbidity in comparison with traditional approaches of open posterior spinal fusion with osteotomy—procedures which many of our patients may not tolerate or even survive. XLX ACR provides us the ability to maximize lordosis segmentally and customize the optimal amount of overall lordosis based on patient-specific alignment goals using iGA through a minimally disruptive approach.”
The NuVasive XLX Interbody System is intended for use in the lumbar spine, from L1 to S1, for the treatment of symptomatic disc degeneration or degenerative spondylolisthesis at one or two adjacent levels, including thoracic disc herniation. It can be used as an adjunct to fusion in patients diagnosed with multilevel degenerative scoliosis.
“With the FDA 510(k) clearance of XLX ACR, we broaden our expandable portfolio to the lateral market,” said Matt Link, executive vice president of strategy, technology and corporate development at NuVasive. “This clearance highlights our continued investment in innovation and Lateral Single-Position Surgery to transform clinical outcomes by developing and evolving spine’s leading solutions and systems to give surgeons the tools they need to best serve their patients.”
The XLX ACR implant will join the existing expandable interbody portfolio of TLX™ and MLX® as NuVasive’s first lateral expandable. TLX and MLX provide customizable correction with streamlined instrumentation from a TLIF approach.
During IMAST 2018, NuVasive is also showcasing LessRay® within its spine solutions portfolio, a foundational technology within Surgical Intelligence. LessRay offers the surgeon and hospital system the opportunity to use significantly reduced radiation imaging in the operating room, while providing unique Image Stitching for surgeons to quickly stitch together fluoroscopic images of any spine segment.
NuVasive will be holding two hands-on workshops at this year’s IMAST that tie in with its innovation in Lateral Single-Position Surgery and LessRay.
NuVasive Hands-on Workshops
- Thursday, July 12 from 12:30—1:30 p.m. PST in Diamond Salon 7, JW Marriott L.A. LIVEProtect yourself and your patients with LessRay: A novel technology to reduce radiation and increase OR efficiency. Presented by Stephen I. Ryu, M.D. and Amer Samdani, M.D.
- Friday, July 13 from 12:00—1:00 p.m. PST in Diamond Salon 7, JW Marriott L.A. LIVELeading. Expanding. Advancing. Insights to Lateral Procedural Solutions. Presented by Christopher R. Brown, M.D. and Jeff Lehmen, M.D.
IMAST 2018 attendees are encouraged to stop by NuVasive booth #32 to learn more about XLX ACR, LessRay and the Company’s comprehensive, industry-leading solutions for creating better clinical outcomes for spine procedures.
About NuVasive
NuVasive, Inc. (NASDAQ: NUVA) is the leader in spine technology innovation, focused on transforming spine surgery and beyond with minimally disruptive, procedurally-integrated solutions designed to deliver reproducible and clinically-proven surgical outcomes. The Company’s portfolio includes access instruments, implantable hardware, biologics, software systems for surgical planning, navigation and imaging solutions, magnetically adjustable implant systems for spine and orthopedics, and intraoperative monitoring service offerings. With over $1 billion in revenues, NuVasive has an approximate 2,400 person workforce in more than 40 countries serving surgeons, hospitals and patients. For more information, please visit www.nuvasive.com.
Forward-Looking Statements
NuVasive cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause NuVasive’s results to differ materially from historical results or those expressed or implied by such forward-looking statements. The potential risks and uncertainties which contribute to the uncertain nature of these statements include, among others, risks associated with acceptance of the Company’s surgical products and procedures by spine surgeons, development and acceptance of new products or product enhancements, clinical and statistical verification of the benefits achieved via the use of NuVasive’s products (including the iGA platform), the Company’s ability to effectually manage inventory as it continues to release new products, its ability to recruit and retain management and key personnel, and the other risks and uncertainties described in NuVasive’s news releases and periodic filings with the Securities and Exchange Commission. NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made.
SOURCE NuVasive, Inc.