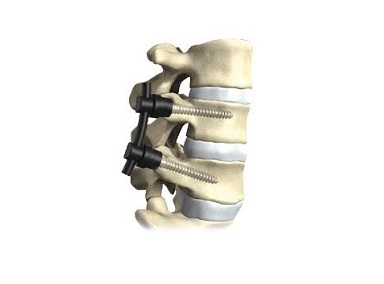
WARSAW, Ind., July 31, 2018 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (NASDAQ:KIDS), a company exclusively focused on advancing the field of pediatric orthopedics, is pleased to announce that FIREFLY® Pedicle Screw Navigation Guides (“FIREFLY”) received additional FDA 510(k) clearance for an expanded indication to include S2AI [screw] trajectory for sacral-iliac fixation in complex spinal reconstruction surgeries, including scoliosis. When applied to the challenging S2AI trajectory, the precise and personalized mechanical guidance enabled by FIREFLY minimizes complexities associated with crossing the sacral-iliac joint while eliminating the need for excessive radiation/fluoroscopy.
OrthoPediatrics is the exclusive distributor of FIREFLY technology in children’s hospitals in the US. FIREFLY is FDA-cleared for use in adult and pediatric populations and is manufactured by Mighty Oak Medical.
The patient-specific, 3D printed FIREFLY Pedicle Screw Navigation Guides are a novel solution to complex and costly navigation systems. This patented navigation technology is designed to increase OR efficiency and eliminate the need for intraoperative radiation, making it the optimal choice in more complicated spinal construct cases, such as those involving the S2AI trajectory.
David Bailey, Executive Vice President of OrthoPediatrics, commented, “We are pleased our partners at Mighty Oak Medical have received additional FDA clearance for this enhancement of the FIREFLY technology. This signifies another pediatric patient segment that surgeons now can treat efficiently and effectively. Ensuring our customers and their patients have access to this novel technology is one more way we continue leading innovation in pediatric orthopedics.”
About Mighty Oak Medical
Mighty Oak Medical is an independent incubator focused on developing and marketing spinal technologies that improve operating room efficiencies, surgical outcomes, and the overall patient experience, by leveraging the talents of experienced surgeons and biomedical engineers. They are located in Englewood, Colorado. For more information, call 720-398-9703 or send an inquiry to info@mightyoakmedical.com.
About OrthoPediatrics Corp.
Founded in 2006, OrthoPediatrics is an orthopedic company focused exclusively on providing a comprehensive product offering to the pediatric orthopedic market to improve the lives of children with orthopedic conditions. OrthoPediatrics currently markets 25 surgical systems that serve three of the largest categories within the pediatric orthopedic market. This offering spans trauma & deformity, scoliosis, and sports medicine/other procedures. OrthoPediatrics’ global sales organization is focused exclusively on pediatric orthopedics and distributes its products in the United States and 38 countries outside the United States.
Investor Contacts
The Ruth Group
Tram Bui / Emma Poalillo
(646) 536-7035 / 7024
tbui@theruthgroup.com / epoalillo@theruthgroup.com