SAN DIEGO, June 20, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, today announced the results of the study “Early Outcomes of Anterior Cervical Discectomy and Fusion Using Porous PEEK™ Interbody Fusion Device,” published online in the Journal of Spine & Neurosurgery, which concludes that NuVasive patented porous polyetheretherketone (PEEK) technology is a clinically viable alternative for improving osseointegration and fusion rates of interbody implants to treat degenerative cervical disc disease.1
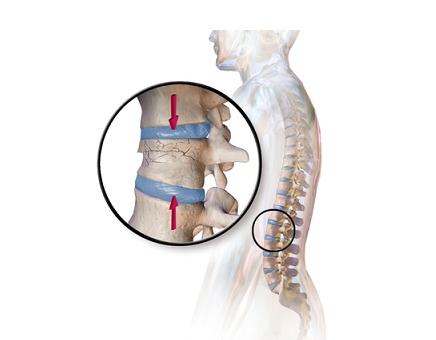
In the study, 50 patients (31 women and 19 men) with an average age of 60 with degenerative cervical disc disease underwent anterior cervical discectomy and fusion using a Cohere® cervical interbody fusion device from NuVasive—with 100 percent of participants experiencing positive results.
Cohere provides a unique three-dimensional Porous PEEK architecture to help elicit and encourage bone ingrowth without compromising implant strength. NuVasive incorporated the technology, which had been validated with 10 years of scientific research, into its portfolio with its acquisition of Vertera Spine in September 2017.
“This interbody fusion device with Porous PEEK surface technology provided improved osseointegration and supported spinal fusion in single level and multi-level cervical fusions,” said Dr. J. Kenneth Burkus of the Hughston Clinic in Columbus, Ga. “Twelve-month clinical outcomes have demonstrated the efficacy and stability of the Cohere Porous PEEK interbody device and shown that it is a clinically viable implant alternative for achieving successful clinical and radiological outcomes in cervical spine fusion surgery, particularly in multi-level or revision surgeries where fusion rates are lower.”
During the course of the clinical study, there were 11 1-level, 23 2-level and 16 3-level fusions between C3 and C7. Patients came from all walks of life and with various comorbidities. Thirteen of them had previously had an unsuccessful anterior cervical fusion procedure. It is notable that all patients who had revision surgery with Cohere achieved fusion six months after the surgery.
At 12 months post-operative ACDF surgery, all 50 patients demonstrated improved motion, decreased neck pain and average disc height increased by more than 4 mm. In addition, each of them showed radiographic fusion.
“NuVasive is the only company in the industry able to offer Porous PEEK technology so that surgeons can deliver life-changing clinical outcomes,” said Matt Link, executive vice president, strategy, technology and corporate development for NuVasive. “The fact that all 50 patients in the clinical study experienced fusion is a testament to the enhanced osseointegration as well as biomechanical and imaging properties of Cohere and Porous PEEK technology, the entire NuVasive Advanced Materials Science™ portfolio, which also includes Modulus® titanium interbody implants, represents the future of porous implant technologies.”
About NuVasive
NuVasive, Inc. (NASDAQ: NUVA) is the leader in spine technology innovation, focused on transforming spine surgery and beyond with minimally disruptive, procedurally-integrated solutions designed to deliver reproducible and clinically-proven surgical outcomes. The Company’s portfolio includes access instruments, implantable hardware, biologics, software systems for surgical planning, navigation and imaging solutions, magnetically adjustable implant systems for spine and orthopedics, and intraoperative monitoring service offerings. With over $1 billion in revenues, NuVasive has an approximate 2,400 person workforce in more than 40 countries serving surgeons, hospitals and patients. For more information, please visit www.nuvasive.com.
Forward-Looking Statements
NuVasive cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause NuVasive’s results to differ materially from historical results or those expressed or implied by such forward-looking statements. The potential risks and uncertainties which contribute to the uncertain nature of these statements include, among others, risks associated with acceptance of the Company’s surgical products and procedures by spine surgeons, development and acceptance of new products or product enhancements, clinical and statistical verification of the benefits achieved via the use of NuVasive’s products (including the iGA® platform), the Company’s ability to effectually manage inventory as it continues to release new products, its ability to recruit and retain management and key personnel, and the other risks and uncertainties described in NuVasive’s news releases and periodic filings with the Securities and Exchange Commission. NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made.
1 Burkus, J. (2018). Early outcomes of anterior cervical discectomy and fusion using porous PEEK interbody fusion device. Journal of Spine & Neurosurgery. Doi: 10.4172/2325-9701.1000295
SOURCE NuVasive, Inc.