LEESBURG, Va., Sept. 26, 2018 (GLOBE NEWSWIRE) — K2M Group Holdings, Inc. (NASDAQ:KTWO) (the “Company” or “K2M”), a global leader of complex spine and minimally invasive solutions focused on achieving three-dimensional Total Body Balance™, today announced that it will showcase its first-to-market MOJAVE™ PL 3D Expandable Interbody System featuring Lamellar 3D Titanium Technology™ and several of its newest spinal solutions at the North American Spine Society 33rd Annual Meeting (NASS) in Los Angeles, CA, September 26-29 (Booth #2001). The Company also announced that MOJAVE PL 3D Expandable received a 2018 Spine Technology Award from Orthopedics This Week, which recognizes the best new spine technologies, engineering teams and inventors for 2018, and in the process, rewards excellence in innovation.
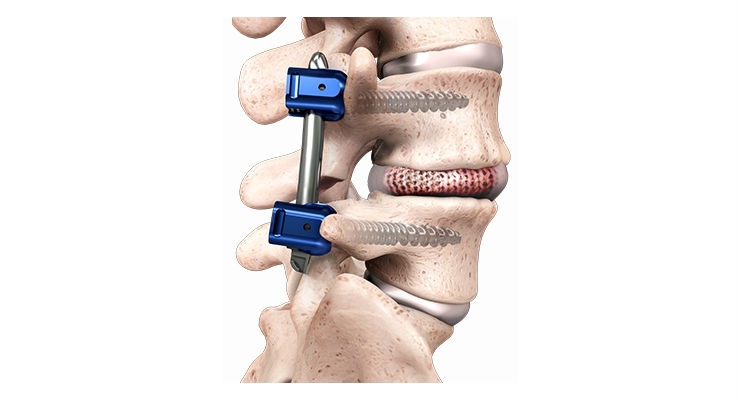
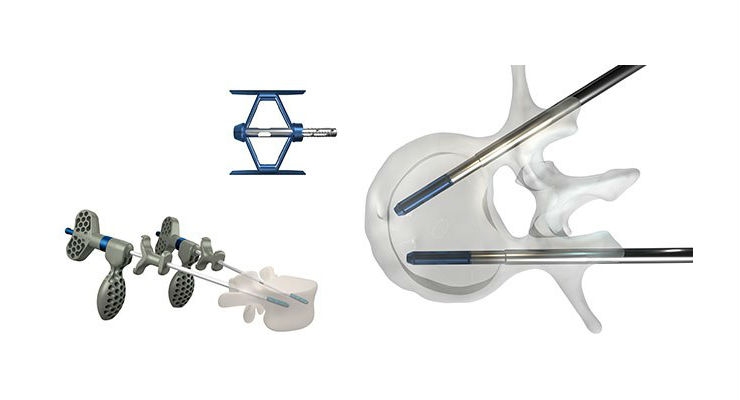
The MOJAVE PL 3D Expandable Interbody System is a first-of-its-kind fusion device allowing for independent control of the anterior and posterior heights in the lumbar spine—a new capability not available with any other product on the market today. Featuring infinite adjustment within its expansion range, the implant can be locked at any desired height and lordosis to help restore sagittal balance. Designed with Lamellar 3D Titanium Technology, MOJAVE PL 3D Expandable incorporates a porous structure and rough surfaces to allow for bony integration throughout the endplates.
At NASS, K2M will also showcase its YUKON™ OCT Spinal System, OZARK™ Guide and View Cervical Plate Systems and comprehensive Balance ACS® (BACS®) Platform. BACS provides surgical solutions focused on achieving balance of the spine by addressing each anatomical vertebral segment with a 360-degree approach of the axial, coronal, and sagittal planes, emphasizing Total Body Balance as an important component of surgical success.
“We are excited to feature our MOJAVE PL 3D Expandable Interbody System, the world’s first 3D-printed fusion device featuring infinite adjustment tools that let surgeons more precisely match the implant to a patient’s anatomy, at this year’s NASS meeting,” said K2M Chairman, President, and CEO Eric Major. “Being recognized by Orthopedics This Week with a Spine Technology Award reflects our commitment to developing innovative solutions that ultimately help improve life for people with spinal diseases across the globe.”
The MOJAVE PL 3D Expandable Interbody System is K2M’s fourth device to win a Spine Technology Award. Orthopedics This Week recognized its 3D-printed CASCADIA™ Interbody Systems in 2016; its MESA® Deformity Cricket and SERENGETI® Minimally Invasive Retractor System were recognized in 2010 and 2009 respectively.
For more information about the MOJAVE PL 3D Expandable Interbody System and K2M and Balance ACS, visit www.K2M.com and www.BACS.com.
About K2M
K2M Group Holdings, Inc. is a global leader of complex spine and minimally invasive solutions focused on achieving three-dimensional Total Body Balance. Since its inception, K2M has designed, developed, and commercialized innovative complex spine and minimally invasive spine technologies and techniques used by spine surgeons to treat some of the most complicated spinal pathologies. K2M has leveraged these core competencies into Balance ACS, a platform of products, services, and research to help surgeons achieve three-dimensional spinal balance across the axial, coronal, and sagittal planes, with the goal of supporting the full continuum of care to facilitate quality patient outcomes. The Balance ACS platform, in combination with the Company’s technologies, techniques and leadership in the 3D-printing of spinal devices, enable K2M to compete favorably in the global spinal surgery market. For more information, visit www.K2M.com and connect with us on Facebook, Twitter, Instagram, LinkedIn and YouTube.
Forward-Looking Statements
The foregoing contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We intend for these forward-looking statements to be covered by the safe harbor provisions of the federal securities laws relating to forward-looking statements. These forward-looking statements include statements relating to the expected timing, completion and effects of the proposed merger, as well as other statements representing management’s beliefs about, future events, transactions, strategies, operations and financial results, including, without limitation, our expectations with respect to the costs and other anticipated financial impacts of the merger; future financial and operating results of K2M Group Holdings, Inc. (“K2M”); K2M’s plans, objectives, expectations and intentions with respect to future operations and services; required approvals to complete the merger by our stockholders and by governmental regulatory authorities, and the timing and conditions for such approvals; the stock price of K2M prior to the consummation of the transactions; and the satisfaction of the closing conditions to the proposed merger. Such forward-looking statements often contain words such as “assume,” “will,” “anticipate,” “believe,” “predict,” “project,” “potential,” “contemplate,” “plan,” “forecast,” “estimate,” “expect,” “intend,” “is targeting,” “may,” “should,” “would,” “could,” “goal,” “seek,” “hope,” “aim,” “continue” and other similar words or expressions or the negative thereof or other variations thereon. Forward-looking statements are made based upon management’s current expectations and beliefs and are not guarantees of future performance. Such forward-looking statements involve numerous assumptions, risks and uncertainties that may cause actual results to differ materially from those expressed or implied in any such statements. Our actual business, financial condition or results of operations may differ materially from those suggested by forward-looking statements as a result of risks and uncertainties which include, among others, those risks and uncertainties described in any of our filings with the Securities and Exchange Commission (the “SEC”). Certain other factors which may impact our business, financial condition or results of operations or which may cause actual results to differ from such forward-looking statements are discussed or included in our periodic reports filed with the SEC and are available on our website at http://www.k2m.com under “Investor Relations.” You are urged to carefully consider all such factors. Although it is believed that the expectations reflected in such forward-looking statements are reasonable and are expressed in good faith, such expectations may not prove to be correct and persons reading this communication are therefore cautioned not to place undue reliance on these forward-looking statements which speak only to expectations as of the date of this communication. We do not undertake or plan to update or revise forward-looking statements to reflect actual results, changes in plans, assumptions, estimates or projections, or other circumstances occurring after the date of this communication, even if such results, changes or circumstances make it clear that any forward-looking information will not be realized. If we make any future public statements or disclosures which modify or impact any of the forward-looking statements contained in or accompanying this communication, such statements or disclosures will be deemed to modify or supersede such statements in this communication.
Additional Information and Where to Find It
This communication does not constitute an offer to buy or sell or the solicitation of an offer to buy or sell any securities or a solicitation of any vote or approval. This communication relates to a proposed acquisition of K2M by Stryker Corporation. In connection with this proposed acquisition, K2M plans to file one or more proxy statements or other documents with the SEC. This communication is not a substitute for any proxy statement or other document K2M may file with the SEC in connection with the proposed transaction. INVESTORS AND SECURITY HOLDERS OF K2M ARE URGED TO READ THE PROXY STATEMENT AND OTHER DOCUMENTS THAT MAY BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Any definitive proxy statement(s) (if and when available) will be mailed to stockholders of K2M. Investors and security holders will be able to obtain free copies of these documents (if and when available) and other documents filed with the SEC by K2M through the website maintained by the SEC at http://www.sec.gov. Copies of the documents filed with the SEC by K2M will be available free of charge on K2M’s internet website at http://www.k2m.com or upon written request to: Secretary, K2M Group Holdings, Inc., 600 Hope Parkway, SE, Leesburg, Virginia 20175, or by telephone at (703) 777-3155.
Participants in Solicitation
K2M, its directors and certain of its executive officers may be considered participants in the solicitation of proxies in connection with the proposed transaction. Information regarding the persons who may, under the rules of the SEC, be deemed participants in such solicitation in connection with the proposed merger will be set forth in the proxy statement if and when it is filed with the SEC. Information about the directors and executive officers of K2M is set forth in its Annual Report on Form 10-K for the fiscal year ended December 31, 2017, which was filed with the SEC on March 1, 2018, its proxy statement for its 2018 annual meeting of stockholders, which was filed with the SEC on April 20, 2018, its Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2018 and June 30, 2018, which were filed with the SEC on May 2, 2018 and August 2, 2018, respectively, and its Current Reports on Form 8-K or Form 8-K/A, which were filed with the SEC on January 8, 2018, January 9, 2018, February 28, 2018, March 29, 2018, May 1, 2018, June 11, 2018, June 14, 2018, June 18, 2018, August 1, 2018 and August 30, 2018.
These documents can be obtained free of charge from the sources indicated above. Additional information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the proxy statement and other relevant materials to be filed with the SEC when they become available.
K2M Group Holdings, Inc.
600 Hope Parkway, SE
Leesburg, Virginia 20175
Tel. (703) 777-3155
www.k2m.com
Media Contact:
Zeno Group on behalf of K2M Group Holdings, Inc.
Christian Emering, 212-299-8985
Christian.Emering@ZenoGroup.com
Investor Contact:
Westwicke Partners on behalf of K2M Group Holdings, Inc.
Mike Piccinino, CFA, 443-213-0500
K2M@westwicke.com