FARMINGDALE, N.Y., Oct. 31, 2016 /PRNewswire/ — Misonix, Inc. (NASDAQ: MSON), an international surgical device company that designs, manufactures and markets innovative therapeutic ultrasonic products for spine surgery, neurosurgery, wound debridement, skull based surgery, laparoscopic surgery and other surgical applications, recently participated in the North American Spine Society (NASS) 2016 Annual Meeting in Boston, MA from October 26-29.
NASS is a global multidisciplinary medical society that utilizes education, research and advocacy to foster the highest quality, ethical, and evidence-based spine care. The NASS 2016 Annual Meeting represented the largest spine meeting and exhibit in the world. It was attended by thousands of spine surgeons gathered to discuss new innovative options, trends, and outcomes in spine surgery.
While at the Misonix exhibit meeting attendees engaged with leading surgeons, including Dr. Nicholas Renaldo, Medical Director of Spine Surgery at Vassar Brothers Medical Center, Poughkeepsie NY; Dr. Eric Woodard, Chief of the Section of Neurosurgery, New England Baptist Hospital, Boston MA; and Dr. Connor Telles from Sierra Pacific Orthopedics in Fresno, CA, to learn about their surgical experiences with the BoneScalpel.
Misonix also hosted – by invitation only – several leading surgeons in the “Innovation Room” where the invited surgeons met with members of the Misonix engineering group to learn more about future innovations under development. Gaining feedback directly from the surgeons at the event is a critical step in assuring that the next generation technologies under development reflect end-user recommendations.
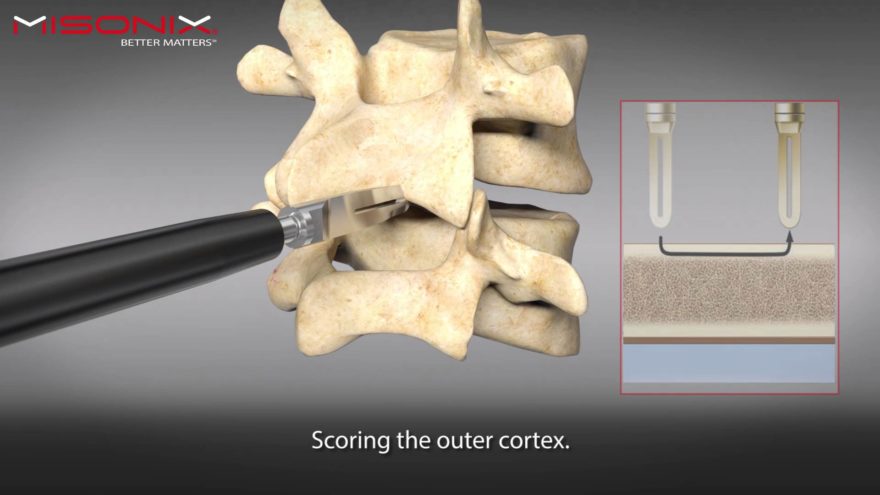
In addition to the Company’s booth presence, Misonix hosted a BoneScalpel hands-on cadaveric workshop entitled, Ultrasonic BoneScalpel Techniques in Complex Spine facilitated by Isador Lieberman, MD, Director of the Scoliosis and Spine Tumor Center at the Texas Back Institute in Plano, TX. Dr. Lieberman demonstrated his clinical usage of the BoneScalpel with attendees having the opportunity for trialing the BoneScalpel ultrasonic bone-cutting instrument. More than 35 spine surgeons were trained on the use of BoneScalpel at the lab.
Commenting further on the week’s events was Dr. Juan Uribe, University of South Florida, Tampa, FL, who presented his experience with the BoneScalpel at Friday’s NASS Solutions Showcase. “The advantages of less blood loss, precise cuts, savings in operating room time, and less hand fatigue are among the many reasons that BoneScalpel is now a requirement for a high percentage of the cases I currently perform. In fact, BoneScalpel has changed the way I practice spine surgery,” added Dr. Uribe.
Stavros Vizirgianakis, interim chief executive officer of Misonix, said, “We are gratified that these key opinion leaders in the spinal and neurosurgical space have adopted our technology into their practices and enthusiastically share their expertise and experiences with others in their profession. This is a very positive reflection on the efficacy of the BoneScalpel, our ultrasonic technology, and the skill sets of these surgical professionals to produce improved patient outcomes, and to do so, in a very efficient manner. We are pleased to be associated with these leading surgical professionals.”
Dr. Isadore Lieberman, who facilitied the BoneScalpel cadaveric workshop, said, “I was extremely pleased with the turnout for the workshop and the continued high level of interest in the BoneScalpel by my peers and colleagues at this year’s NASS meeting. In my opinion, BoneScalpel continues to be one of the most important innovations in spine surgery in the past several years and given the number of attendees who participated in the workshop this year, clearly people are taking notice of the importance of this technology.”
Misonix Senior Vice President of Global Sales and Marketing, Scott Ludecker, commented, “BoneScalpel’s ability to mitigate the potential for soft tissue collateral damage and minimize blood loss during complex spine surgeries are some of the key benefits which have drawn the spinal surgeon community to BoneScalpel. These attributes are universally understood as being cornerstones for better patient outcomes. Events like this week’s hands-on cadaver workshop are idea forums for surgeons to experience for themselves the benefits of our technology. Having leading surgeons like Dr. Lieberman available to share his personal experience with them is invaluable.”
About Misonix
Misonix, Inc. designs, develops, manufactures and markets therapeutic ultrasonic medical devices. Misonix’s therapeutic ultrasonic platform is the basis for several innovative medical technologies. Addressing a combined market estimated to be in excess of $1.5 billion annually; Misonix’s proprietary ultrasonic medical devices are used in spine surgery, neurosurgery, orthopedic surgery, wound debridement, cosmetic surgery, laparoscopic surgery, and other surgical and medical applications. Additional information is available on the Company’s Web site at www.misonix.com.
Safe Harbor Statement
With the exception of historical information contained in this press release, content herein may contain “forward looking statements” that are made pursuant to the Safe Harbor Provisions of the Private Securities Litigation Reform Act of 1995. These statements are based on management’s current expectations and are subject to uncertainty and changes in circumstances. Investors are cautioned that forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from the statements made. These factors include general economic conditions, delays and risks associated with the performance of contracts, risks associated with international sales and currency fluctuations, uncertainties as a result of research and development, acceptable results from clinical studies, including publication of results and patient/procedure data with varying levels of statistical relevancy, risks involved in introducing and marketing new products, potential acquisitions, consumer and industry acceptance, litigation and/or court proceedings, including the timing and monetary requirements of such activities, the timing of finding strategic partners and implementing such relationships, regulatory risks including approval of pending and/or contemplated 510(k) filings, the ability to achieve and maintain profitability in the Company’s business lines, and other factors discussed in the Company’s Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. The Company disclaims any obligation to update its forward-looking relationships.
Logo – http://photos.prnewswire.com/prnh/20160201/328020LOGO
SOURCE Misonix, Inc.
Related Links
http://www.misonix.com