IRVINE, Calif., Oct. 4, 2016 /PRNewswire-iReach/ — joimax®, the global acting German developer and marketer of technologies and training methods for endoscopic minimally invasive spinal surgery, will again exhibit at Eurospine 2016 taking place from October 5 – 7 in Berlin. During the conference, joimax®will launch two new products – the MultiZYTE® SI Endoscopic Sacroiliac Joint Therapy Set and its new Intracs® Interaoperative Navigation Tracking & Control System.
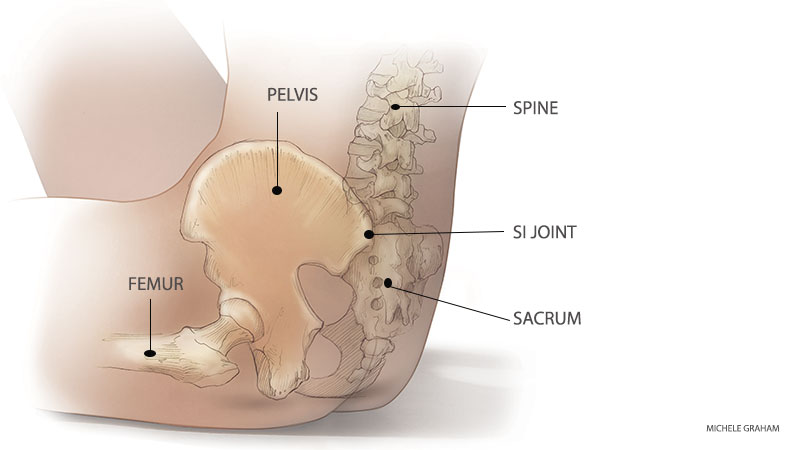
MultiZYTE® SI Endoscopic Sacroiliac Joint Therapy is developed for the treatment of the Sacroiliac Joint Syndrome (SIJS), which has been rediscovered as a major back pain generator in recent years. This kind of pain is commonly felt in the lower back, the gluteal region, the hip and thigh and can even radiate into the leg and foot. Until the 1930s, SI-Joint was the main reason for low back pain, but since 1934 the focus has been on disc herniation. According to recent studies, 15 to 25% of all low back pain is caused by the SI-Joint and up to 43% in patients having undergone lumbosacral fusion. MultiZYTE® SI joimax® now offers a well developed and lasting treatment option for this disease.
The Intracs® Interaoperative Navigation Tracking & Control System was developed in cooperation between joimax® GmbH Karlsruhe and fiagon GmbH in Berlin, both located in Germany. With the Intracs® system, joimax® instruments can be navigated directly at the tip using Fiagons “chip at the tip” technology which allows tracking of instruments with a diameter in the 1mm range. The Intracs ® Navigation and Monitor Unit is integrated in the joimax® Endoscopic Tower and all necessary tools are tailored for the TESSYS® (transforaminal) and iLESSYS® (interlaminar) procedures as well as for minimally invasive stabilization procedures, like EndoLIF® and Percusys®. The main features of the Intracs® system are needle navigation through Vector-Tip-Target processing tools, the merging of 2D X-Rays with 3D- images of a patient’s CT or MRI scans, and the constant orientation control of any endoscope used in joimax procedures.
“With these two new developments we are in the position to enhance our endoscopic minimally-invasive product portfolio, driving adoption rates to meet new heights. With the world unique Intracs® system, navigation is now faster, safer and more accurate than ever before using an electromagnetic (EM) field. Radiation exposure for both, patients and surgeons will be significantly reduced,” says Wolfgang Ries, CEO and founder of joimax®. “Additionally the learning curve for all spinal endoscopic procedures offered by joimax® will now be significantly reduced and the rapid expansion of all these techniques is now well layed-out,” he continues.
About joimax®
Founded in Karlsruhe, Germany, in 2001, joimax® is the leading developer and marketer of complete systems for endoscopic minimally invasive spinal surgery. With TESSYS® (transforaminal), iLESSYS® (interlaminar) and CESSYS® (cervical) for decompression procedures, MultiZYTE® RT (e.g. for rhizotomy) and with MultiZYTE® SI for SI-Joint therapy or with EndoLIF®and Percusys® for minimally-invasive endoscopic assisted stabilizations, proven endoscopic systems are provided that, together, cover a whole variety of indications.
In procedures for herniated disc, stenosis, pain therapy or spinal stabilization treatment, surgeons utilize joimax® technologies to operate through small incisions – under local or full anesthetic – via tissue and muscle-sparing corridors through natural openings into the spinal canal (e.g. intervertebral foramen, the “Kambin triangle”). For more information visit www.joimax.com
Media Contact: Melissa Brumley, joimax® Inc., 001 949 859 3472, Melissa.brumley@joimaxusa.com
News distributed by PR Newswire iReach: https://ireach.prnewswire.com
SOURCE joimax