ALACHUA, Fla.–(BUSINESS WIRE)–RTI Surgical, Inc. (Nasdaq: RTIX), a global surgical implant company, announced the full commercial launch of its Fortilink®-TS and -L IBF Systems, adding to a growing series of interbody fusion devices featuring proprietary TETRAfuse® 3D Technology. The Fortilink-TS and -L Systems are intended for use in lumbar interbody fusion procedures at one or two adjoining levels in patients with degenerative disc disease. TETRAfuse 3D Technology is the first 3D printed polymer-based interbody fusion device to incorporate a nano-roughisurface that has demonstrated, in a pre-clinical study, more notable trabecular bone ingrowth compared to PEEK and titanium-coated PEEKii.
“The Fortilink-L System is the first osteointegrative, radiolucent cage,” said Joseph O’Brien, M.D., M.P.H., founder of the Washington Spine & Scoliosis Institute at OrthoBethesda in Bethesda, Maryland, and one of the first surgeons to implant the Fortilink-L device. “While other companies are focusing on titanium, TETRAfuse 3D Technology offers ingrowth features that help the implant to fully integrate with the fusing bone. It also has best-in-class X-ray compatibility, which allows me to track my patients’ progress with more confidence.”
John O’Toole, M.D., M.S., Professor of Neurosurgery at Rush University Medical Center in Chicago, Illinois, and one of the first surgeons to implant the Fortilink-TS device, added, “The introduction of TETRAfuse 3D Technology revolutionizes the interbody device market by combining a favorable modulus of elasticity, optimal imaging characteristics and a surface design promoting vigorous bone ingrowth. I believe TETRAfuse 3D Technology meets all the criteria for an interbody through enhanced biology, biomechanics and ease of use to help improve outcomes for patients.”
The Fortilink-L IBF System is intended for a lateral transpsoas approach to the lumbar spine, also known as lateral lumbar interbody fusion (LLIF) surgeries. The Fortilink-TS System is intended for traditional posterior approaches known as transforaminal lumbar interbody fusion (TLIF) or bilateral posterior lumbar interbody fusion (PLIF) surgeries. TETRAfuse 3D Technology features a nano-roughi surface with antibacterial characteristics†iii and is designed to participate in fusionii without compromising mechanical integrityi or radiographic visibility.i
“Based on the positive feedback from our surgeon customers and its unique design, we believe TETRAfuse 3D Technology is a new frontier in spine surgery,” said Camille Farhat, President and CEO, RTI Surgical. “RTI is committed to ongoing clinical research and development of innovative spine-focused solutions that meet the demands of surgeons and improve patient outcomes.”
RTI commercially launched its first implant using TETRAfuse 3D Technology, the Fortilink-C IBF System, in October 2017. It is used as an interbody fusion device in anterior cervical discectomy and fusion (ACDF) surgeries. All Fortilink implants have received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
For more information about the Fortilink series of devices and TETRAfuse 3D Technology, visit www.tetrafuse3D.com.
About Degenerative Disc Disease (DDD)
Degenerative disc disease (DDD) is an age-related condition when one or more discs between the vertebrae of the spinal column deteriorate or break down.iv A symptom of DDD can include low back pain (LBP)iv, which is the most common musculoskeletal condition affecting adults and the leading cause of disability worldwide.v Approximately 40 percent of adults suffer from chronic low back pain at some point in their lifetimevi, and health care costs and productivity losses associated with it are more than $50 billion annually.vii Spinal fusion surgery is considered a viable treatment option for reducing pain and improving function in individuals diagnosed with DDD and LBP, who have not found relief with non-surgical care.viii
About RTI Surgical, Inc.
RTI Surgical is a leading global surgical implant company providing surgeons with safe biologic, metal and synthetic implants. Committed to delivering a higher standard, RTI’s implants are used in sports medicine, general surgery, spine, orthopedic and trauma procedures and are distributed in nearly 50 countries. RTI has four manufacturing facilities throughout the U.S. and Europe. RTI is accredited in the U.S. by the American Association of Tissue Banks and is a member of AdvaMed. For more information, please visit www.rtix.com.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management’s current expectations, estimates and projections about our industry, our management’s beliefs and certain assumptions made by our management. Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” variations of such words and similar expressions are intended to identify such forward-looking statements. In addition, except for historical information, any statements made in this communication about anticipated financial results, growth rates, new product introductions, future operational improvements, gaining market share and results or regulatory actions or approvals or changes to agreements with distributors also are forward-looking statements. These statements are not guarantees of future performance and are subject to risks and uncertainties, including the risks described in public filings with the U.S. Securities and Exchange Commission (SEC). Our actual results may differ materially from the anticipated results reflected in these forward-looking statements. Copies of the company’s SEC filings may be obtained by contacting the company or the SEC or by visiting RTI’s website at www.rtix.com or the SEC’s website at www.sec.gov.
i Data on file at RTI Surgical, Inc.
ii Data on file at RTI Surgical, Inc. Performance data from animal studies may not be representative of performance in humans.
iii Wang M, Bhardwaj B, Webster T; Antibacterial properties of PEKK for orthopedic applications. Int’l Journal of Nanomedicine. 2017: 12 6471-6476. iv Donally C, Dulebohn S. Lumbar Degenerative Disk Disease. StatPearls. Oct 2017. Online at https://www.ncbi.nlm.nih.gov/books/NBK448134/. Last accessed May 25, 2018.
v Allegri M, Montella S, Salici F, et al. Mechanisms for low back pain: a guide for diagnosis and therapy. F1000Research. Oct 2016. Online at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4926733/. Last accessed May 25, 2018.
vi Manchikanti L, Singh V, Falco FJ, et al. Epidemiology of low back pain in adults. Neuromodulation. 2014 Oct;17 Suppl 2:3-10. Online at https://www.ncbi.nlm.nih.gov/pubmed/25395111. Last accessed May 25, 2018.
vii Licciardone JC. The epidemiology and medical management of low back pain during ambulatory medical care visits in the United States. Osteopath Med Prim Care. 2008; 2: 11. Online at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2631527/. Last accessed May 25, 2018.
viii Phillips FM, Slosar PJ, Youssef JA et al. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine (Phila Pa 1976). 2013 Apr 1;38(7):E409-22. Online at https://www.ncbi.nlm.nih.gov/pubmed/23334400. Last accessed May 25, 2018.
†Lab data may not be representative of the effects with all bacteria or performance when implanted in humans. Staphyloccocus epidermidis and Pseudomonas aeruginosa were subject bacterial strains in this study.
Please refer to the labeling for clinical applications, warnings, precautions and other instructions for use.
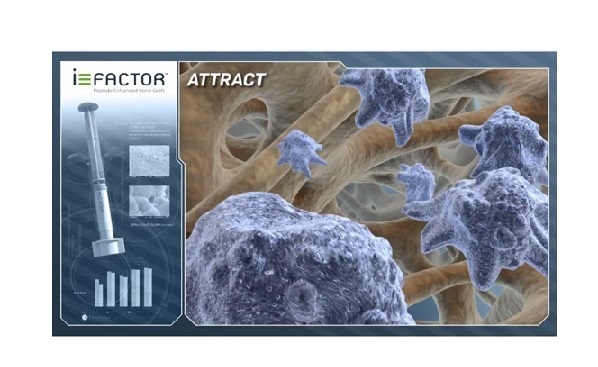
 WESTMINSTER, Colo., June 13, 2018 /PRNewswire/ — Cerapedics, a privately-held orthobiologics company, today announced that it has been selected as a winner in the 2018 MedTech Breakthrough Awards program. The award recognizes the company’s i‑FACTOR™ Peptide Enhanced Bone Graft as the best new surgical technology solution.
WESTMINSTER, Colo., June 13, 2018 /PRNewswire/ — Cerapedics, a privately-held orthobiologics company, today announced that it has been selected as a winner in the 2018 MedTech Breakthrough Awards program. The award recognizes the company’s i‑FACTOR™ Peptide Enhanced Bone Graft as the best new surgical technology solution.