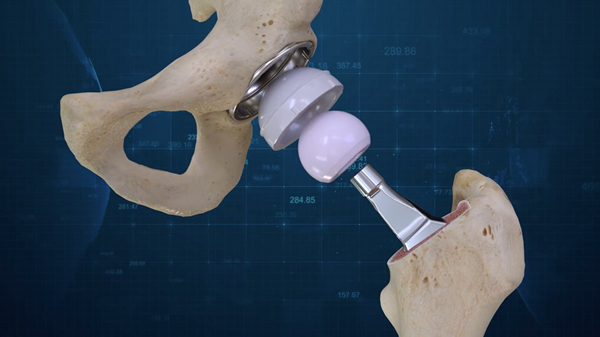
BILLERICA, Mass., Aug. 01, 2018 (GLOBE NEWSWIRE) — Conformis, Inc. (NASDAQ:CFMS), a medical technology company that offers patient conforming joint replacement implants, today announced completion of the first two Conformis Hip System implants. The Conformis Hip System is the first ever 3D designed primary total hip replacement system. The first surgeries were performed by Gregory Martin, M.D., Joint Fellowship Trained Orthopedic Surgeon and founder of Personalized Orthopedics of the Palm Beaches, and took place at a leading HCA Facility, JFK Medical Center in Atlantis, Florida on July 31, 2018.
“Partnering with our expert surgeon design team and leveraging our extensive experience in automated 3D printing and additive manufacturing enabled us to bring a revolutionary new hip replacement system to market. Our 3D implant design process provides surgeons with interactive input and improves operational efficiencies compared to 2D templating. The design process produces specific individualized pre-navigated cutting guides and implants. A groundbreaking acetabular reaming system has also been developed,” said Mark Augusti, chief executive officer and president of Conformis. “With this launch, we expect to lead the way in innovative solutions for the $7B hip replacement market by providing surgeons with game-changing operative solutions to better serve them and their patients both in hospitals and ambulatory surgical settings.”
Similar to the design process for the Conformis Knee technologies, the Conformis Hip System uses proprietary advanced imaging and design software, to design, manufacture and deliver the suite of FDA-cleared patient conforming Knee and Hip replacement implants. After each patient’s CT scan is converted into a 3-dimensional computer model, the unique measurements of each patient’s anatomy are transformed into a comprehensive, individualized, pre-operative surgical plan that is delivered to the surgeon well in advance of the operation. Surgeons are able to collaborate with Conformis during the planning process in order to design the optimal Hip System for each patient based on surgeon preferences.
“The Conformis Hip System is designed to address many of the short comings of primary hip replacement today. For the first time, orthopedic surgeons have a fully-guided system designed to address the wide variations in anatomy presented across our cases,” said Dr. Martin M.D*, a member of the surgeon design team. “The Conformis system builds upon traditional methods for hip replacement surgery with proven materials and components which, today, are offered in only limited standard configurations. Due to the accuracy of the personalized pre-operative surgical plan, the 3D printed patient conforming cutting guides and hip implant components, my hope is that with the new Conformis Hip system, surgeons will be able to improve both patient outcomes and operational efficiencies.”
Each component of the Conformis Hip System is pre-navigated to fit the patient, with certain components designed specifically for that patient. The Conformis Hip System is delivered directly to the hospital or surgery center in a single patient-labeled kit, eliminating the need for excess inventory. Patient conforming, single-use, 3D printed cutting guides are also included, limiting the need for the vast amount of reusable instruments required for a standard off-the-shelf total hip replacement.
The first two Conformis Hip System surgeries were conducted as part of a limited launch. Timing for a complete commercial launch is expected to be announced in 2019.
The global hip joint reconstruction market is projected at over $7B, and about 400,000 total hip replacements are performed in the United States each year.
*Gregory Martin, MD is a consultant to Conformis, Inc.
About Conformis, Inc.
Conformis is a medical technology company that uses its proprietary iFit Image-to-Implant technology platform to develop, manufacture and sell joint replacement implants that are designed and manufactured to fit and conform to each patient’s unique anatomy. Conformis offers a broad line of patient conforming total and partial knee systems and a hip system that include sterilized single-use instruments delivered in a single package to the hospital. Conformis owns or exclusively in-licenses over 400 issued patents and pending patent applications that cover patient-specific implants and instrumentation for all major joints. In clinical studies, Conformis iTotal CR demonstrated superior clinical outcomes, including better function and greater patient satisfaction, compared to traditional, off-the-shelf implants.
For more information, visit www.conformis.com. To receive future releases in e-mail alerts, sign up at http://ir.conformis.com/.
Cautionary Statement Regarding Forward-Looking Statements
Statements in this press release about our future expectations, plans and prospects, including statements about the anticipated timing of our product launches, and our financial position and results, total revenue, product revenue, gross margin, operations, as well as other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” and similar expressions, constitute forward-looking statements within the meaning of the safe harbor provisions of The Private Securities Litigation Reform Act of 1995. You should not place undue reliance on our forward-looking statements. Actual results could differ materially from the expectations disclosed in the forward-looking statements we make as a result of a variety of risks and uncertainties, including risks related to our estimates and expectations regarding our revenue, gross margin, expenses, revenue growth and other results of operations, and the other risks and uncertainties described in the “Risk Factors” sections of our public filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent our views as of the date hereof. We anticipate that subsequent events and developments may cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date hereof.
Media Contact:
Kelly Wakelee
(212)-253-8241
Investor Contact:
Oksana Bradley
(781) 374-5598
A photo accompanying this announcement is available at: http://www.globenewswire.com/NewsRoom/AttachmentNg/e52cfbc4-fc9b-4c4d-b337-1990354e34a7
The photo is also available via AP PhotoExpress.
Source: Conformis, Inc.
This article appears in: News Headlines
Referenced Stocks: CFMS