April 12, 2017
SUNNYVALE, Calif.–(BUSINESS WIRE)–Simplify Medical, Inc., maker of the Simplify® cervical artificial disc, today announced that the first patient has been treated in the device’s pivotal clinical trial studying its use in two adjacent levels of the spine as a treatment for cervical degenerative disc disease. The Simplify Disc is designed to be clearly viewed on magnetic resonance imaging (MRI) without the artifact that can result from metal used in typical spine implants, potentially protecting patients from radiation associated with computed tomography (CT) scans.
Following the first case at Texas Back Institute, Simplify Disc IDE study investigator John Peloza, MD said, “Disc implantation went smoothly and the patient is doing quite well.” Dr. Peloza, medical director of the Center for Spine Care, continued, “I look forward to studying the use of the Simplify Disc at two levels, in collaboration with the Texas Back Institute team. I believe the Simplify Disc’s anatomic design with lower heights can help minimize overdistraction, which may improve outcomes in multi-level procedures.”
Richard Guyer, MD, chairman of the Texas Back Institute Foundation and national co-primary investigator for the study, said, “We are excited to start the Simplify Disc Two-Level IDE study. The device is made of materials designed to optimize its viewing and viewing of the adjacent spinal canal on MRI without artifact. This may eliminate or minimize the use of post-operative CT scans and reduce the risk of the associated radiation to the patient.”
The prospective, randomized controlled Simplify Disc pivotal trial will encompass up to 215 patients at up to 15 centers and will compare cervical implantation of two contiguous discs from C3 to C7 with two-level cervical fusion surgery. The composite primary endpoint includes functional improvement, pain relief and safety. The national co-principal investigators are Domagoj Coric, MD, chief of the department of neurosurgery, Carolinas Medical Center, and Dr. Guyer. For information about eligibility or enrollment in the two-level pivotal trial, please visit http://www.simplifytrial.com/.
While magnetic resonance imaging (MRI) is widely used pre-operatively for surgical planning, spine surgeons often switch to CT post-operatively in order to accommodate metal components, which can make it difficult to view the devices, as well as the facets and adjacent levels. However, CT scans have been shown to expose patients to ionizing radiation that equates to 400 to 550 chest X-rays per scan.
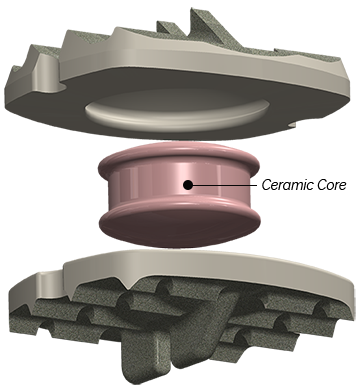
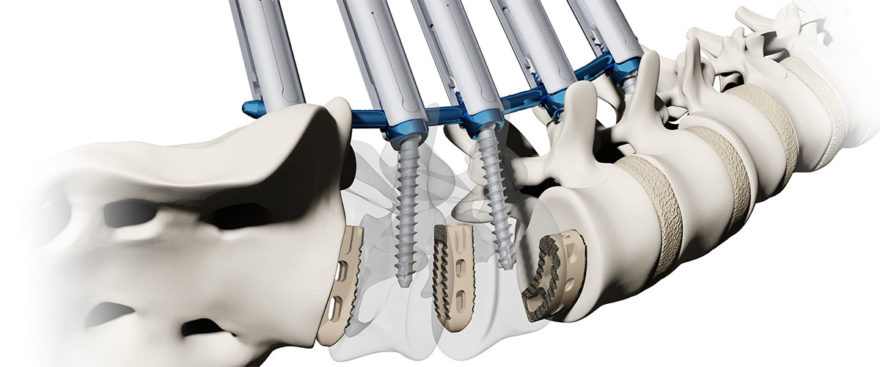
Composed of primarily non-metal materials (PEEK-on-ceramic), the Simplify Disc is designed to be viewed on MRI in order to minimize patient exposure to radiation. With no metal in its articulating components, the disc is also designed for low levels of wear to optimize long-term durability. Implantation of the Simplify Disc is accomplished in a simple, three-step procedure. The Simplify Disc is also anatomically designed with low height implant options to accommodate patients with smaller cervical disc spaces, making it ideal for women and certain regional populations. The device is considered MRI-conditional, posing no known hazard in an MRI environment within prescribed conditions of use.
“We have designed the Simplify Disc to be responsive to surgeons’ needs, providing them with the potential to improve patient outcomes,” said David Hovda, Simplify Medical Chief Executive Officer. “We are enthusiastic about moving forward with the pivotal trial.”
The Simplify Disc is also enrolling patients in a second pivotal trial comparing one-level cervical implantation of the disc between C3 to C7 with cervical fusion surgery from a historical nonconcurrent control group. More information about the one-level clinical trial is available at www.SimplifyTrial.com.
The Simplify Disc has received the CE Mark and has been used to treat more than 600 patients outside the U.S. over the last three years. Early clinical data has shown substantial improvement in patient pain scores and functional improvement after treatment.
ABOUT SIMPLIFY MEDICAL
Simplify Medical is focused on cervical spinal disc arthroplasty, using innovative, MRI-friendly materials designed to decrease the need for ionizing radiation and enhance patient options. Simplify Medical is located in Sunnyvale, California. To learn more, visit http://www.simplifymedical.com/.
Caution: The Simplify Disc is an Investigational device in the United States and is limited by law to investigational use.
Contacts
Chronic Communications, Inc.
Michelle McAdam, (949) 545-6654
michelle@chronic-comm.com