WARSAW, Ind., Feb. 1, 2017 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global leader in musculoskeletal healthcare, today announced that the U.S. Food and Drug Administration (FDA) approved an expanded 26-week efficacy claim for its single-injection viscosupplement Gel-One® Cross-linked Hyaluronate for the treatment of knee pain associated with osteoarthritis. Gel-One Cross-linked Hyaluronate may be administered in the physician office setting and is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to non-pharmacologic therapy, non-steroidal anti-inflammatory drugs (NSAIDs) or simple analgesics, e.g., acetaminophen1.
“Gel-One Hyaluronate is a vital component in the continuum of treatments for knee osteoarthritis, offering millions of patients the potential for up to six months of pain relief with a single injection,” said David Nolan, Zimmer Biomet Group President, Biologics, Extremities, Sports Medicine, Surgical, Trauma, Foot and Ankle, Office Based Technologies and Zimmer Biomet Signature Solutions. “The expanded efficacy claim not only strengthens our competitive positioning, but reinforces our commitment to alleviating pain and restoring mobility for patients at every stage in the continuum of musculoskeletal care, including conservative and non-surgical options.”
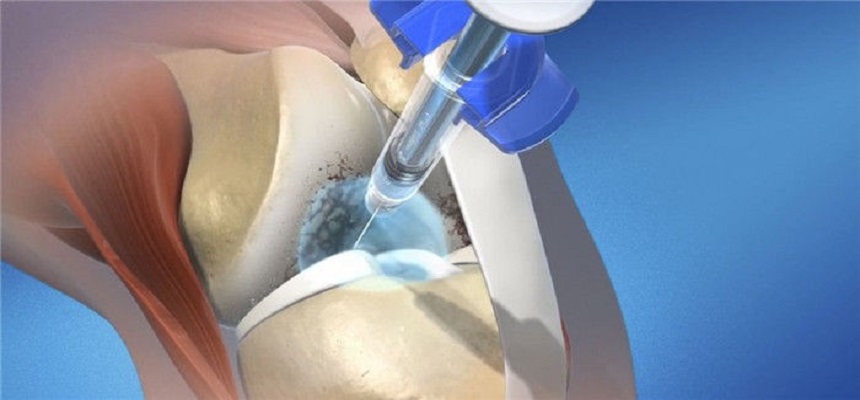
Gel-One Cross-Linked Hyaluronate is the first low-volume viscosupplement available in a single-injection formulation for the treatment of osteoarthritis of the knee. Hyaluronic acid (HA) products such as Gel-One Cross-linked Hyaluronate may supplement the natural HA of the knee, which provides cushioning and lubrication to the joint. Gel-One Cross-linked Hyaluronate requires only a single 3 mL injection to complete the treatment course.
About Zimmer Biomet
Founded in 1927 and headquartered in Warsaw, Indiana, Zimmer Biomet is a global leader in musculoskeletal healthcare. We design, manufacture and market orthopaedic reconstructive products; sports medicine, biologics, extremities and trauma products; office based technologies; spine, craniomaxillofacial and thoracic products; dental implants; and related surgical products.
We collaborate with healthcare professionals around the globe to advance the pace of innovation. Our products and solutions help treat patients suffering from disorders of, or injuries to, bones, joints or supporting soft tissues. Together with healthcare professionals, we help millions of people live better lives.
We have operations in more than 25 countries around the world and sell products in more than 100 countries. For more information, visit zimmerbiomet.com or follow Zimmer Biomet on Twitter at twitter.com/zimmerbiomet.
Cautionary Statement Regarding Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning Zimmer Biomet’s expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties that could cause actual outcomes and results to differ materially. For a list and description of some of such risks and uncertainties, see our periodic reports filed with the SEC. These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in Zimmer Biomet’s filings with the SEC. We disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be set forth in our periodic reports. Accordingly, such forward-looking statements speak only as of the date made. Readers of this news release are cautioned not to place undue reliance on these forward-looking statements, since, while management believes the assumptions on which the forward-looking statements are based are reasonable, there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this news release.
1Important Safety Information
Important Safety Information
Before using Gel-One Hyaluronate, tell your doctor if you are allergic to hyaluronan products, cinnamon, or products from birds such as feathers, eggs, and poultry. Gel-One Hyaluronate is only for injection into the knee, performed by a doctor or other qualified health care professional. You should not receive Gel-One Hyaluronate injection if you have a skin disease or infection around the area where the injection will be given. Gel-One Hyaluronate has not been tested to show pain relief in joints other than the knee and for conditions other than OA. Gel-One Hyaluronate has not been tested in patients who are pregnant, mothers who are nursing, or anyone under the age of 21. You should tell your doctor if you think you are pregnant or if you are nursing a child. Talk to your doctor before resuming strenuous or prolonged weight-bearing activities after treatment. The effectiveness of repeat treatment cycles of Gel-One Hyaluronate has not been established. The side effects most commonly seen after injection of Gel-One Hyaluronate in the clinical trial were knee pain, swelling, and/or fluid build-up around the knee. These reactions are generally mild and do not last long. Other conditions, including but not limited to skin redness and rash, knee stiffness, knee muscular weakness and dizziness, were also reported. If any of these symptoms or signs appear after you are given Gel-One Hyaluronate or if you have any other problems, you should call your doctor. Ask your surgeon if you are a candidate and discuss potential risks. For additional information, call 1-800-348-2759, or visit www.zimmerbiomet.com.
This material is intended for US patients and US Health Care Professionals.
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/zimmer-biomet-announces-fda-approval-of-expanded-26-week-efficacy-claim-for-gel-one-cross-linked-hyaluronate-for-treatment-of-pain-associated-with-knee-osteoarthritis-300399479.html
SOURCE Zimmer Biomet Holdings, Inc.
News Provided by Acquire Media