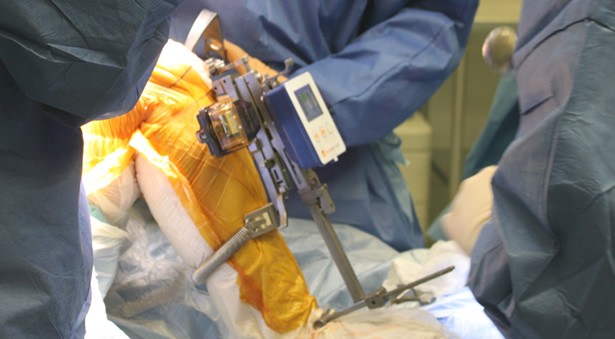
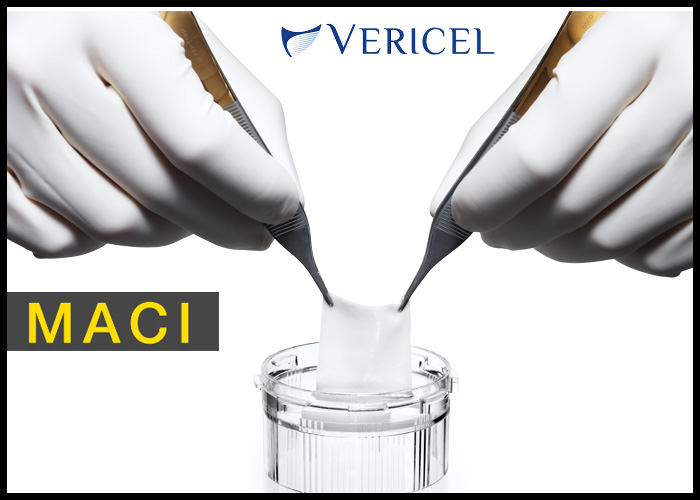
CAMBRIDGE, Mass., Feb. 01, 2017 (GLOBE NEWSWIRE) — Vericel Corporation (NASDAQ:VCEL), a leading developer of expanded autologous cell therapies for the treatment of patients with serious diseases and conditions, today announced the first implant of MACI®(autologous cultured chondrocytes on porcine collagen membrane) in the United States for the repair of symptomatic single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults.
“While surgeons have long understood that autologous chondrocyte implantation can repair cartilage tissue, the previous surgical procedure was more invasive, technically demanding, time consuming and could result in an uneven distribution of cells,” said Dr. David Recker, chief medical officer of Vericel. “With the introduction of MACI, surgeons now have an FDA-approved product in which the patient’s own cells can be reproducibly delivered using less invasive techniques. The MACI implant not only ensures more uniform distribution of the cells in the cartilage defect, but also greatly simplifies the implantation procedure.”
“Treating the first patient with MACI this soon following FDA approval is incredibly rewarding for everyone at Vericel,” said Nick Colangelo, president and CEO of Vericel. “We believe that the simplified MACI surgical technique, together with the MACI Phase 3 clinical data demonstrating statistically significantly greater improvement in pain and function scores compared to microfracture, will drive significant growth for our cartilage repair franchise. We are now focused on realizing this growth potential by providing training to expand the number of implanting surgeons and working with payers to ensure reimbursement for MACI.”
About MACI
MACI® (autologous cultured chondrocytes on porcine collagen membrane) is an autologous cellular scaffold product that is indicated for the repair of symptomatic single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults. The MACI implant consists of autologous cultured chondrocytes seeded onto a resorbable Type I/III collagen membrane. Autologous cultured chondrocytes are human-derived cells which are obtained from the patient’s own cartilage for the manufacture of MACI.
The FDA approval of MACI was supported by the results of SUMMIT trial1, a Phase 3 two‑year, prospective, multicenter, randomized, open-label, parallel-group study that enrolled a total of 144 patients, ages 18 to 54 years, with at least one symptomatic Outerbridge Grade III or IV focal cartilage defect on the medial femoral condyle, lateral femoral condyle, and/or the trochlea. The co-primary efficacy endpoint was change from baseline to Week 104 for the subject’s Knee injury and Osteoarthritis Outcome Score (KOOS) in 2 subscales: Pain and Function (Sports and Recreational Activities [SRA]).2 At Week 104, KOOS pain and function (SRA) had improved from baseline in both treatment groups, but the improvement was statistically significantly (p<0.001) greater in the MACI group compared with the microfracture group. In a responder analysis, the proportion of subjects with at least a 10‑point improvement in both KOOS pain and function (SRA) was greater in the MACI group (63/72 = 87.5%; 95% CI [77.6%, 94.6%]) compared with the microfracture group (49/72 = 68.1%; 95% CI [56.0%, 78.6%]).
The most frequently occurring adverse reactions (≥5%) reported for MACI in the 2‑year randomized, controlled clinical trial were arthralgia, tendonitis, back pain, joint swelling, and joint effusion. Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis.
Important Safety Information
- MACI is contraindicated in patients with a known history of hypersensitivity to gentamicin, other aminoglycosides, or products of porcine or bovine origin. MACI is also contraindicated for patients with severe osteoarthritis of the knee, inflammatory arthritis, inflammatory joint disease, or uncorrected congenital blood coagulation disorders. MACI is also not indicated for use in patients who have undergone prior knee surgery in the past six months, excluding surgery to procure a biopsy or a concomitant procedure to prepare the knee for a MACI implant.
- MACI is contraindicated in patients who are unable to follow a physician-prescribed post-surgical rehabilitation program.
- The safety of MACI in patients with malignancy in the area of cartilage biopsy or implant is unknown. Expansion of present malignant or dysplastic cells during the culturing process or implantation is possible.
- Patients undergoing procedures associated with MACI are not routinely tested for transmissible infectious diseases. A cartilage biopsy and MACI implant may carry the risk of transmitting infectious diseases to healthcare providers handling the tissue. Universal precautions should be employed when handling the biopsy samples and the MACI product.
- Final sterility test results are not available at the time of shipping. In the case of positive sterility results, health care provider(s) will be contacted.
- To create a favorable environment for healing, concomitant pathologies that include meniscal pathology, cruciate ligament instability and joint misalignment, must be addressed prior to or concurrent with the implantation of MACI.
- Local treatment guidelines regarding the use of thromboprophylaxis and antibiotic prophylaxis around orthopaedic surgery should be followed. Use in patients with local inflammations or active infections in the bone, joint, and surrounding soft tissue should be temporarily deferred until documented recovery.
- The MACI implant is not recommended during pregnancy. For implantations post-pregnancy, the safety of breast feeding to infant has not been determined.
- Use of MACI in pediatric patients or patients over 55 years of age has not been assessed.
- The most frequently occurring adverse reactions reported for MACI (≥5%) were arthralgia, tendonitis, back pain, joint swelling, and joint effusion.
- Serious adverse reactions reported for MACI were arthralgia, cartilage injury, meniscus injury, treatment failure, and osteoarthritis.
About Articular Cartilage Defects of the Knee
Articular cartilage is a highly organized avascular tissue composed of chondrocytes embedded within an extracellular matrix of collagens, proteoglycans and noncollagenous proteins. Its primary function is to enable the smooth articulation of joint surfaces, and to cushion compressive, tensile and shearing forces. Articular cartilage damage is caused by both acute and repetitive trauma resulting in knee pain, effusion or mechanical symptoms such as catching and locking, and swelling. Since articular cartilage is avascular it has little capacity to repair itself or regenerate. Articular cartilage lesions that are left untreated may progress to debilitating joint pain, dysfunction, and osteoarthritis.3 The prevalence rate for cartilage lesions in the knee has been reported to be 63% in patients undergoing investigational arthroscopies.4
About Vericel Corporation
Vericel develops, manufactures, and markets expanded autologous cell therapies for the treatment of patients with serious diseases and conditions. The company currently markets two cell therapy products in the United States. Carticel® (autologous cultured chondrocytes) is an autologous chondrocyte implant for the treatment of cartilage defects in the knee in patients who have had an inadequate response to a prior arthroscopic or other surgical repair procedure. Epicel® (cultured epidermal autografts) is a permanent skin replacement for the treatment of patients with deep dermal or full thickness burns greater than or equal to 30% of total body surface area. Vericel is marketing MACI® (autologous cultured chondrocytes on porcine collagen membrane), an autologous cellularized scaffold product indicated for the repair of symptomatic, single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults, which has just been approved by the FDA. Vericel is also developing ixmyelocel‑T, an autologous multicellular therapy intended to treat advanced heart failure due to ischemic dilated cardiomyopathy (DCM). For more information, please visit the company’s website at www.vcel.com.
Epicel®, Carticel®, and MACI® are registered trademarks of Vericel Corporation. © 2017 Vericel Corporation. All rights reserved.
This document contains forward-looking statements, including, without limitation, statements concerning anticipated progress, objectives and expectations regarding the commercial potential of MACI® and our other products, and timing, and objectives and expectations regarding our company described herein, all of which involve certain risks and uncertainties. These statements are often, but are not always, made through the use of words or phrases such as “anticipates,” “intends,” “estimates,” “plans,” “expects,” “we believe,” “we intend,” and similar words or phrases, or future or conditional verbs such as “will,” “would,” “should,” “potential,” “can continue,” “could,” “may,” or similar expressions. Actual results may differ significantly from the expectations contained in the forward-looking statements. Among the factors that may result in differences are the inherent uncertainties associated with competitive developments, clinical trial and product development activities, regulatory approval requirements, estimating the commercial potential of our products and product candidates, market demand for our products, product performance and our ability to supply or meet customer demand for our products. These and other significant factors are discussed in greater detail in Vericel’s Annual Report on Form 10-K for the year ended December 31, 2015, filed with the Securities and Exchange Commission (“SEC”) on March 14, 2016, Quarterly Reports on Form 10-Q and other filings with the SEC. These forward-looking statements reflect management’s current views and Vericel does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this release except as required by law.
References
1Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014 Jun;42(6):1384-94.
2Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64.
3Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994‑1009.
4Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60.
CONTACT: Chad Rubin The Trout Group crubin@troutgroup.com (646) 378-2947 or Lee Stern The Trout Group lstern@troutgroup.com (646) 378-2922





 Download as PDF
Download as PDF