Schlieren (Zurich), Switzerland, June 19, 2018
Kuros Biosciences will this week present results from several investigator-led clinical case studies of MagnetOs Granules at the 15th annual State of Spine Surgery Think Tank, a leading conference uniquely dedicated to innovation in spinal surgery.
The case studies, in which MagnetOs Granules were implanted in the spine, were performed by Alwyn Jones MB ChB, BSc, MSc, FRCS, FRSC (Orth), Consultant Orthopaedic Spinal Surgeon at Spire Cardiff Hospital in the UK.
The key clinical outcomes at six months were improved back and leg pain. The most important fusion outcomes were good incorporation of MagnetOs in the posterior fusion bed, graft resorption and remodelling to bone, and progression towards fusion.
Key takeaways from the case studies were:
- MagnetOs Granules were well‐tolerated and no device related adverse events were reported in the small cohort of patients requiring spinal fusion
- MagnetOs Granules were easy to apply as a stand‐alone graft or when mixed with bone marrow aspirate (BMA) or local bone
- Resorption and remodelling of MagnetOs Granules was evident from as early as 3 months post‐implantation
- MagnetOs Granules promoted spinal fusion in a mixed cohort of patients when implanted using 5 different surgical approaches
Alwyn Jones said: “I implanted MagnetOs in a cohort of five patients requiring spinal fusion in 2017 and I’m very pleased with their progress. In all cases, my radiographic data indicated remodelling of MagnetOs and progression towards a fusion. All five patients had a reduction in pain, improvement in disability index and improved clinical symptoms compared to their pre-surgical assessment. This initial experience has encouraged me to use MagnetOs in my broader clinical practice.”
Joost de Bruijn, Chief Executive Officer of Kuros, said: “The results from these investigator-led clinical case studies with MagnetOs are very gratifying and underline the interest of the clinical research community in our CE marked product. The MagnetOs Granules were easy to apply and showed clear clinical benefits for patients, giving them an improved quality of life with less pain.”
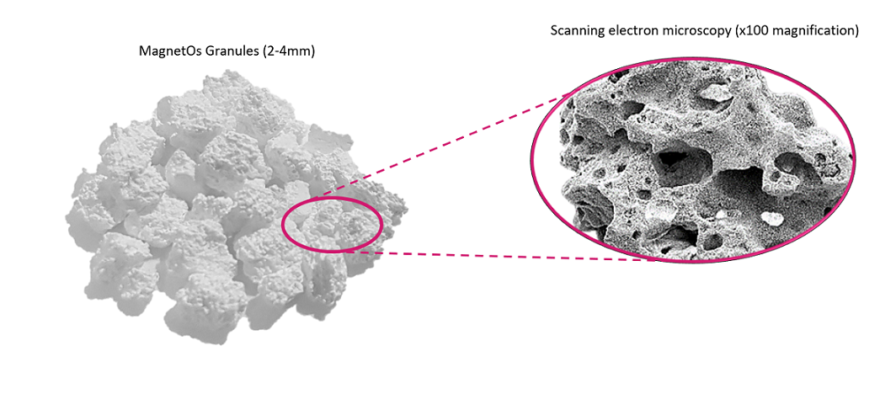
MagnetOs promotes local bone formation equivalent to current gold standard, autograft. MagnetOs is a bone graft substitute intended to fill bony voids or gaps of the human skeletal system and promote the formation of bone at the implanted site. A substantial number of clinically relevant and predictive studies have demonstrated its equivalence to the current gold standard (patient’s own bone, which may not be available in sufficient quantities and/or involves morbidity, costs and pain associated with its harvesting from another healthy site of the patient’s body). MagnetOs is a bone graft comprising biphasic calcium phosphate with an advanced submicron surface topography that directs bone formation after implantation. With its unique submicron surface topography, MagnetOs preferentially directs early wound healing toward the bone-forming pathway, resulting in an osteoinductive claim in Europe. MagnetOs is available as granules and as a putty formulation.
The State of Spine Surgery Think Tank, formerly known as the Cabo Meeting, takes place June 21-13 in Aruba.
For further information, please contact:
|
Kuros Biosciences AG |
Media & Investors |
About Kuros Biosciences AG
Kuros Biosciences (SIX:KURN) is focused on the development of innovative products for bone regeneration and is located in Schlieren (Zurich), Switzerland and Bilthoven, The Netherlands. Visit www.kurosbio.com for additional information on Kuros, its people, science and product pipeline.
Forward Looking Statements
This media release contains certain forward-looking statements that involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. You are urged to consider statements that include the words “will” or “expect” or the negative of those words or other similar words to be uncertain and forward-looking. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward-looking statements include scientific, business, economic and financial factors, Against the background of these uncertainties, readers should not rely on forward-looking statements. The Company assumes no responsibility for updating forward-looking statements or adapting them to future events or developments.
Kuros Biosciences Ltd, Wagistrasse 25, CH-8952 Schlieren