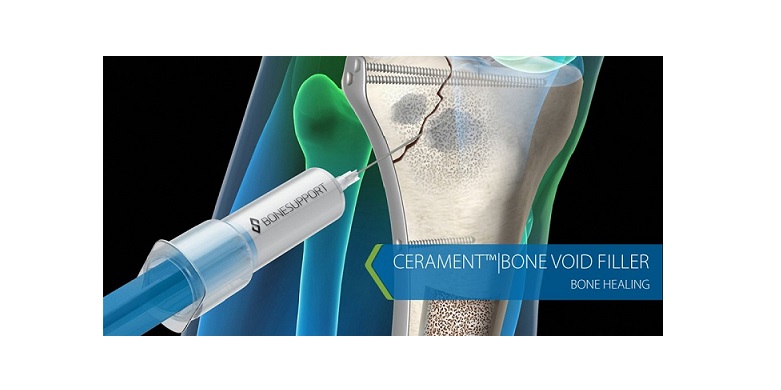
Lund, Sweden, 13.00 CET, 24 May 2018 – BONESUPPORT, announces an agreement with Collagen Matrix, Inc. that will allow it to expand its US offering with products that are complementary to CERAMENT BVF. BONESUPPORT will start selling and marketing selected Collagen Matrix bone graft substitute products under its own brand name, and through its own US distribution network in Q4 2018.
Emil Billbäck, CEO of BONESUPPORT said, “Today’s deal with Collagen Matrix is important to our plans to build a larger, faster growing US business under our own control. The Collagen Matrix products can be osteoinductive and osteogenic when combined with bone marrow aspirate making them complementary to CERAMENT BVF, which is osteoconductive. We intend to launch these products under our own brand name later this year giving us a suite of products that will allow us to meet more of the bone graft substitute needs of the orthopedic surgeon. Establishing a broader portfolio of bone graft substitute technologies will also significantly improve our competitive position with payors including GPO/IDN’s (Group Purchasing Organization/Integrated Delivery Network), and help our new distribution channel to be more competitive.”
Collagen Matrix products, when combined with autogenous bone marrow, are both osteoinductive and osteogenic. They are used to fill bony voids or gaps of the skeletal system caused by surgery or traumatic injury.
On 17 May 2018, BONESUPPORT announced that it intended to create its own optimal US commercial platform by:
- Building its own network of independent distributors that will be key in driving the sales of BONESUPPORT’s products, to a broad range of orthopedic indications.
- Growing the size and capabilities of its own US commercial organization, which will increase from 14 to around 23 employees by the end of 2018
- Extending its product offering based on new formulations of CERAMENT as well as synergistic bone graft substitute products via strategic collaborations such as the one announced with Collagen Matrix today.
For more information contact:
Emil Billbäck, CEO
+46 (0) 46 286 53 70
Björn Westberg, CFO
+46 (0) 46 286 53 60
Citigate Dewe Rogerson
Pip Batty, David Dible, Shabnam Bashir, Isabelle Andrews
+44 (0)20 7282 1022
bonesupport@citigatedewerogerson.com
About Collagen Matrix Inc.
Collagen Matrix, Inc., founded in 1997, delivers a full line of the highest-quality collagen and mineral based medical devices that support the body’s natural ability to regenerate. The Company currently manufactures finished medical devices in the areas of Dental, Spine, Orthopaedic, Dural Repair and Nerve Repair Surgery. The evolution of the Company’s leadership, proprietary technologies, manufacturing expertise and product portfolio has established a solid foundation for continued growth.
More information about Collagen Matrix can be found at www.CollagenMatrix.com
About BONESUPPORT™
BONESUPPORT is an innovative and rapidly growing commercial stage orthobiologics company, based in Lund, Sweden. The Company develops and commercializes innovative injectable bio-ceramic bone graft substitutes that remodel to the patient’s own bone and have the capability of eluting drugs directly into the bone void.
BONESUPPORT’s marketed bio-ceramic bone graft substitutes CERAMENT® BONE VOID FILLER (BVF), CERAMENT® G* and CERAMENT® V* are all based on the Company’s novel and proprietary CERAMENT technology platform.
The Company’s products are targeting a large addressable market opportunity across trauma, chronic osteomyelitis (bone infection), revision arthroplasty (replacement of a joint prosthesis) and infected diabetic foot.
BONESUPPORT’s total sales increased from SEK 62 million in 2015 to SEK 129 million in 2017, representing a compound annual growth rate of 45%.
The Company’s research and development is focused on the continuing development and refinement of its CERAMENT technology to extend its use into additional indications by the elution of other drugs and therapeutic agents. The Company currently has a pipeline of pre-clinical product candidates that have been designed to promote bone growth.
BONESUPPORT is listed on Nasdaq Stockholm and trades under the ticker “BONEX” (ISIN code: SE0009858152). Further information is available at www.bonesupport.com
*CERAMENT G: Not available in the United States, for investigational use only. CERAMENT V: Not available in the United States
BONESUPPORT™ and CERAMENT® are registered trademarks.
This information is such information as BONESUPPORT HOLDING AB (publ) is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication, through the agency of the contact person set out above, at 13.00 CET on 24 May 2018.