LONDON, Nov. 2, 2017 /PRNewswire/ —
Shoulder Arthroplasty Market by Procedure (Partial Shoulder Arthroplasty, Total Shoulder Arthroplasty, and Revision Shoulder Arthroplasty), Device (Shoulder Arthroplasty Resurfacing Implants, Shoulder Arthroplasty Trauma Devices, and Shoulder Arthroplasty Platform Systems), Indication (Arthritis, Fracture/Dislocation, Rotator Cuff Tear Arthropathy, Hill Sachs Defect, and Other), and End User (Hospitals & Clinics, and Outpatient Surgical Centers) – Global Opportunity Analysis and Industry Forecast, 2017-2023
Download the full report: https://www.reportbuyer.com/product/5139386
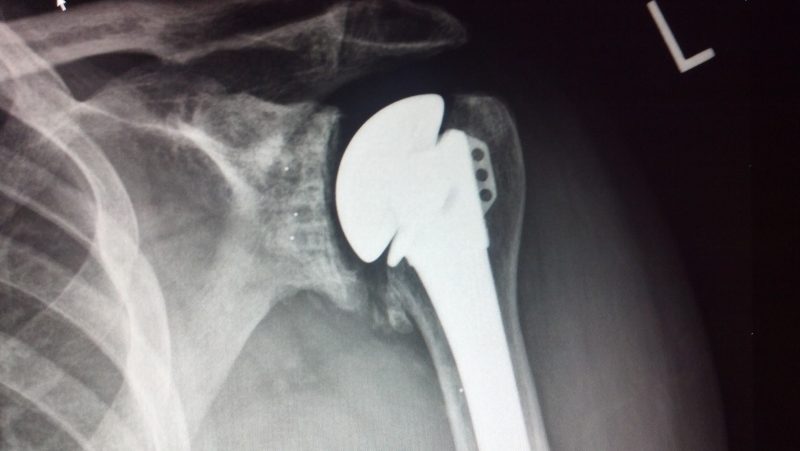
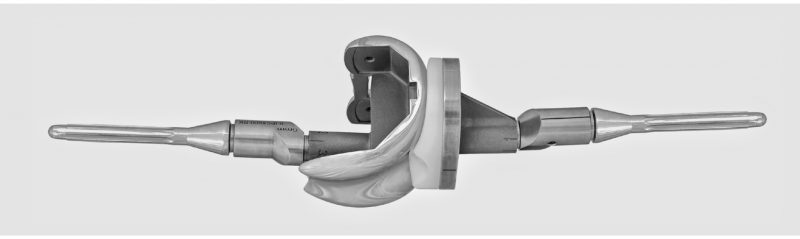
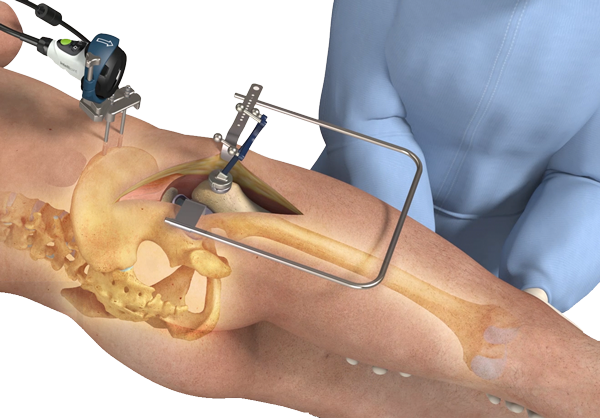
Shoulder arthroplasty or shoulder replacement is a surgical procedure, which assists in the replacement of damaged ball and socket joint located in the shoulder. This is done using a prosthesis, usually consists of polythene and metal components. Shoulder arthroplasty has become a preferred modality of surgeons for the treatment of osteoarthritis, rheumatoid arthritis, and osteonecrosis, as these procedures have yielded significant and reproducible results. Large-scale adoption of total shoulder arthroplasty procedures for the treatment of people suffering from arthritis has highly contributed to the market growth.
Increase in prevalence of shoulder arthritis and rise in geriatric population worldwide drive the growth of the global shoulder arthroplasty market. In addition, rise in awareness towards shoulder arthroplasties and advancements in the surgical techniques supplement the market growth. However, increase in risk of postoperative complications, such as neurologic injury and hematoma, hampers the market growth. Conversely, rise in investments by the key players to manufacture shoulder arthroplasty devices in the developing economies is expected to offer lucrative opportunities for the market growth. The global shoulder arthroplasty market was valued at $1,080 million in 2016, and is expected to reach $1,814 million by 2023, registering a CAGR of 7.6% from 2017 to 2023.
The shoulder arthroplasty market is segmented based on procedure, device, indication, end user, and region. On the basis of procedure, the market is divided into partial shoulder arthroplasty, total shoulder arthroplasty, and revision shoulder arthroplasty. By device, it is categorized into shoulder arthroplasty resurfacing implants, shoulder arthroplasty trauma devices, and shoulder arthroplasty platform systems. Depending on indication, it is classified into arthritis, fracture/dislocation, rotator cuff tear arthropathy, Hill Sachs defect, and other. As per end user, it is fragmented into hospitals & clinics and outpatient surgical centers. Regionally, the market is analyzed across North America, Europe, Asia-Pacific, and LAMEA.
The total shoulder arthroplasty (TSA) segment accounted for the highest share in the global shoulder arthroplasty market in 2016, and is anticipated to register a highest CAGR of 9.2% from 2017 to 2023. This is attributed to increase in adoption of total reverse shoulder arthroplasty.
In addition, shoulder arthroplasties are majorly performed in hospitals and clinics, and thus this segment generated the highest revenue. In the recent years, Asia-Pacific witnessed rapid growth in the shoulder arthroplasties market, owing to steady increase in the population suffering from various forms of arthritis. Moreover, increase in investments by the key players of Japan and India to develop shoulder arthroplasty devices is projected to offer lucrative opportunities for the expansion of the market.
KEY BENEFITS FOR STAKEHOLDERS
The study provides an in-depth analysis of the global shoulder arthroplasty market with the current trends and future estimations to elucidate the imminent investment pockets.
Comprehensive analysis of the factors that drive and restrict the market growth is provided.
Comprehensive quantitative analysis of the industry is provided from 2016 to 2023 to assist stakeholders to capitalize on the prevailing market opportunities.
Extensive analysis of the key segments of the industry helps to understand the trends in shoulder arthroplasty devices and procedures performed globally.
Key players and their strategies are provided to understand the competitive outlook of the industry.
KEY MARKET SEGMENTS
By Procedure
Partial Shoulder Arthroplasty
Partial Resurfacing
Hemi Resurfacing
Partial-mid Head
Stemmed Hemi
Total Shoulder Arthroplasty
Total Resurfacing
Total-mid Head
Total Conventional
Total Reverse
Revision Shoulder Arthroplasty
By Device
Shoulder Arthroplasty Resurfacing Implants
Shoulder Arthroplasty Trauma Devices
Shoulder Arthroplasty Platform Systems
By Indication
Arthritis
Osteoarthritis
Rheumatoid Arthritis
Other Inflammatory Arthritis
Fracture/Dislocation
Rotator Cuff Tear Arthropathy
Hill Sachs Defect
Other
By End User
Hospitals & Clinics
Outpatient Surgical Centers
By Region
North America
U.S.
Canada
Mexico
Europe
UK
France
Germany
Italy
Spain
Rest of Europe
Asia-Pacific
Japan
China
India
Australia
South Korea
Rest of Asia-Pacific
LAMEA
Brazil
South Africa
Turkey
Saudi Arabia
Rest of LAMEA
KEY MARKET PLAYERS
Wright Medical Group, Inc. /Tornier Inc.
Integra LifeSciences Corporation
Zimmer Biomet
Johnson & Johnson (DePuy Synthes)
Arthrex, Inc.
Smith and Nephew Plc.
Conmed Corporation
DJO Global
Evolutis
Exactech, Inc.
The other players in the value chain include (profiles not included in the report)
Implantcast GmbH
Lima Corporate
Medacta International
Kinamed Incorporated
Corin
Imascap SAS
Catalyst Orthoscience
Biotechni
Cayenne Medical
BioTek Instruments, Inc.
Download the full report: https://www.reportbuyer.com/product/5139386
About Reportbuyer
Reportbuyer is a leading industry intelligence solution that provides all market research reports from top publishers
https://www.reportbuyer.com
For more information:
Sarah Smith
Research Advisor at Reportbuyer.com
Email: query@reportbuyer.com
Tel: +44 208 816 85 48
Website: www.reportbuyer.com
SOURCE ReportBuyer