March 06, 2018
NEW ORLEANS–(BUSINESS WIRE)–Medacta® International, the privately held family-owned global leader in the design of innovative joint replacement and spine surgery products, today launched its new MOTO™ Medial Partial Knee System, Medacta Shoulder System and a third offset option for its MasterLoc™ Hip System at the American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting, held March 6-10 in New Orleans, Louisiana. The company will be showcasing these products at Booth #6329 and will also be co-sponsoring a guest reception honoring Italy’s contributions to orthopedic innovation.
“Medacta remains focused on delivering new and innovative orthopedic solutions paired with hands-on support, solid education programs and expert mentorship to help surgeons more efficiently and effectively meet their patients’ needs,” said Francesco Siccardi, executive vice president of Medacta International. “With our MOTO Knee, Shoulder System and MasterLoc LAT PLUS, we’re making it easier for surgeons to treat complex cases or patients with hard-to-match anatomies and optimizing procedures for the outpatient setting to meet growing demand.”
MOTO Partial Knee System: Enabling Superior Fit, Balance and Alignment
Medacta’s MOTO Medial Partial Knee System is a compartment-specific, fixed-bearing solution for partial knee replacement, or unicompartmental knee arthroplasty (UKA), in which only a portion of the knee is resurfaced. Partial knee replacements are rapidly becoming a common choice for orthopedic surgeons seeking to offer patients the option of pain relief in the outpatient setting. With patient demand on the rise, Medacta designed the MOTO System and detailed education platform to simplify common procedural challenges and make partial knee cases easier to perform in an outpatient setting.
The MOTO implants are designed for each specific compartment using an anthropometric database of more than 45,000 CT and MRI knee scans to optimize anatomic coverage, contours, size range and precise fit. Perhaps the most distinguishing feature of MOTO is the instrumentation that allows for precise bone resections to balance knee flexion-extension gap mismatch, while maintaining slight alignment under-correction.
“There was still much to be improved in partial knee arthroplasty,” said J. Mandume Kerina, MD, founder of Unova Health Clinic in Lady Lake, Florida and co-designer of the MOTO Partial Knee, who has been performing outpatient knee replacements for more than a decade. “Based on registry data and results, the MOTO Partial Knee was designed following the fixed-bearing, round-on-flat design, but improves upon the options and flexibility built into the system. The result – an anatomic design, optimized sizing options, logical instrumentation and a versatile surgical technique – gives surgeons more control than before and better addresses challenging patient anatomies.”
In keeping with its commitment to surgeon education and training, Medacta created a unique education program around the MOTO Partial Knee that provides top-level medical education and continuous support to ensure surgeons transitioning to using the implant have adequate support and are able to deliver the best possible outcomes to their patients.
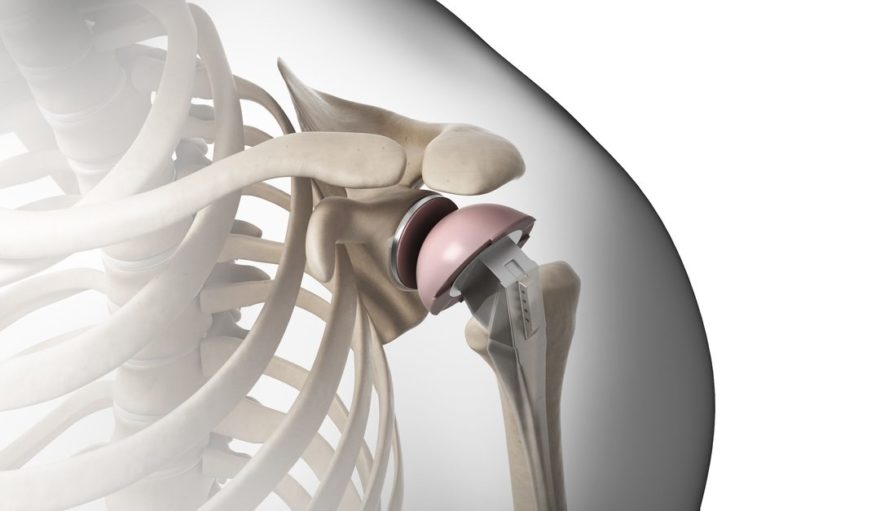
Medacta Shoulder System: Featuring Anatomic and Reverse Options for Complex Cases
The Medacta Shoulder System is a modular solution designed to address the unique challenges around complex shoulder arthritis. Developed by an international team of expert surgeons, the platform offers both Anatomic and Reverse Shoulders to deliver the modularity and compatibility demanded by today’s marketplace. Its unparalleled implant selection allows for an anatomic fit for a wide range of pathologies, with other options including wide-ranging sizes, adjustable offsets and other innovative configurations.
Medacta’s first foray in the shoulder surgery market, the platform was unveiled in February 2017 following its first ever surgery in Europe, while its first U.S. surgery took place in the fall of 2017. The system is now available for surgeons throughout the United States and Europe.
MasterLoc LAT PLUS: Providing Unmatched Offset Range for Optimal Restoration
With the addition of the LAT PLUS, Medacta’s MasterLoc Hip System is the only flat-tapered wedge stem for total hip replacement with a unique progressive triple offset, giving surgeons more options to better suit their patients’ needs without impacting leg length. Adding to the MasterLoc’s existing standard offset (STD) and lateralized version (LAT), which adds an extra six millimeters of femoral offset to the STD, the LAT PLUS offers another five millimeters of offset to help surgeons enhance component stability.
The MasterLoc Hip System was introduced in November 2016 as another addition to Medacta’s cementless implant portfolio. The MasterLoc features Medacta’s Mectagrip plasma-sprayed titanium coating, designed to enhance initial fixation with its high coefficient of friction and the potential long-term stability inherent to titanium plasma-sprayed devices.
Guest Nation Reception at AAOS: Honoring Italy’s Contributions
Medacta International is also co-sponsoring the 2018 Guest Nation Reception honoring Italian orthopedic professional attendees at AAOS. The Boards of Directors of the Italian Society of Orthopaedics and Traumatology (SIOT) and of AAOS will be present among more than 200 guests. The reception will take place on Friday, March 9, 2018 from 6:30-8:30 p.m. Eastern.
About Medacta International
Medacta® International is a world leading manufacturer of orthopedic implants, neurosurgical systems, and instrumentation. Medacta’s revolutionary approach and responsible innovation have resulted in standard of care breakthroughs in hip replacement with the AMIS® system and total knee replacement with MyKnee® patient matched technology. Over the last 10 years, Medacta has grown dramatically by taking a different approach and placing value on all aspects of the care experience from design to training to sustainability. Medacta is headquartered in Castel San Pietro, Switzerland, and operates in over 30 countries. To learn more about Medacta International, please visit www.medacta.com or follow @Medacta on Twitter.
Contacts
For Medacta International
Jill Bongiorni, 516-729-2250
Jill@torchcomllc.com