July 10, 2018
MEMPHIS, Tenn.–(BUSINESS WIRE)–Active Implants LLC, a company that develops orthopedic implant solutions, today announced that the final patient was treated in the two clinical trials evaluating the NUsurface® Meniscus Implant for the treatment of persistent knee pain caused by injured or deteriorated meniscus tissue. The trials are being conducted to evaluate the safety and effectiveness of the NUsurface Meniscus Implant in support of U.S. Food and Drug Administration (FDA) De Novo 510(k) clearance.
“We are now one step closer to filling the gap in treatment options between minimally invasive meniscus repair and total knee replacement, which is a large unmet need in the orthopedic market,” said Ted Davis, president and CEO of Active Implants. “With enrollment complete, we will continue to work closely with the FDA and now focus our efforts on collecting the data required for the U.S. regulatory submission. We thank our investigator surgeons and patients for making this day possible.”
The two clinical trials enrolled a combined 243 patients, 176 of which received the NUsurface Meniscus Implant. The VENUS (Verification of the Effectiveness of the NUsurface System) trial is a randomized, multi-centered, prospective, controlled study comparing the NUsurface Meniscus Implant to the non-surgical standard of care and enrolled 128 patients at 10 U.S. study sites. The SUN (Safety Using NUsurface) trial is a single-arm study assessing the safety and probable benefit of the NUsurface Meniscus Implant in restoring function similar to that of a natural, healthy meniscus and enrolled 115 patients at 13 U.S. study sites. Active Implants conducted the two different types of studies concurrently in order to bring the NUsurface Meniscus Implant to market as quickly as possible while the company worked with the FDA to finalize the regulatory clearance for marketing in the U.S.
“The NUsurface Implant is being studied in patients who still have persistent knee pain following a meniscus surgery, have exhausted other treatment options, and are too old for repair and too young for total knee replacement,” said Elliott Hershman, MD, orthopedic surgeon at Lenox Hill Hospital in New York City and medical director for the studies.
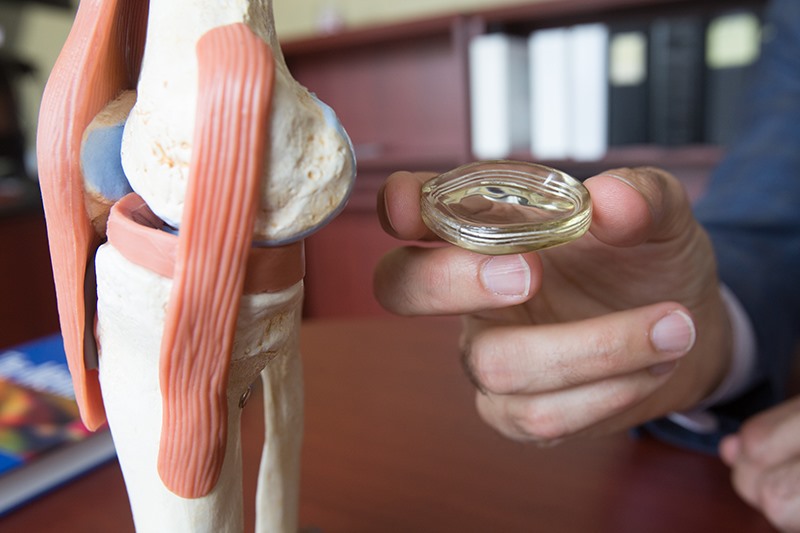
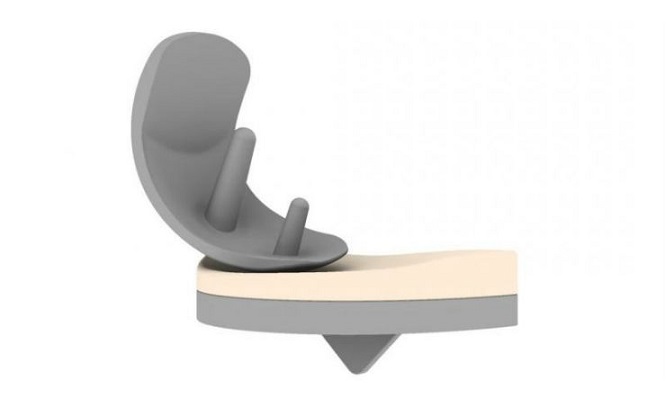
If approved by the U.S. Food & Drug Administration, the NUsurface Implant would be the first “artificial meniscus.” The meniscus is a tissue pad between the thigh and shin bones, and once damaged, the meniscus in middle aged patients has a very limited ability to heal itself. Current treatment for a damaged or torn meniscus includes pain management, physical therapy, injections, repair techniques or meniscectomy. It has been estimated that from 700,000 to over 1 million partial meniscectomies are performed annually in the U.S. in an attempt to alleviate pain; however, recent studies have shown that many people who get a meniscectomy continue to experience pain that impacts their quality of life and can eventually lead to knee replacement surgery.
About the NUsurface® Meniscus Implant
The NUsurface® Meniscus Implant is an investigational treatment for patients with persistent knee pain following medial meniscus surgery. It is made from medical grade polymer and, as a result of its unique materials, composite structure and design, does not require fixation to bone or soft tissues. The NUsurface Meniscus Implant mimics the function of the natural meniscus and redistributes loads transmitted across the knee joint. The NUsurface Meniscus Implant has been used in Europe under CE Mark since 2008 and in Israel since 2011.
About Active Implants LLC
Active Implants LLC develops orthopedic implant solutions that complement the natural biomechanics of the musculoskeletal system, allowing patients to maintain or return to an active lifestyle. Active Implants is privately held with headquarters in Memphis, Tennessee. European offices are in Haarlem, The Netherlands, with R&D facilities in Netanya, Israel. For more information, visit www.activeimplants.com.
CAUTION Investigational device. Limited by United States law to investigational use.
Contacts
Merryman Communications
Joni Ramirez, 323-532-0746
joni@merrymancommunications.com