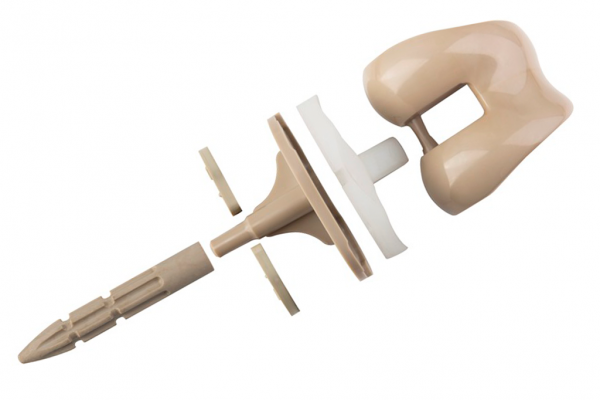
WARSAW, Ind., April 16, 2018 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global leader in musculoskeletal healthcare, today announced the completion of the first surgical case utilizing its Persona® Trabecular Metal™ (TM) Tibia by Dr. Richard Moore, Boise, Idaho on March 20, 2018. The Persona Trabecular Metal Tibia received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in January 2018 and CE Mark approval in April 2018. The Persona TM Tibia is an integral component of the Company’s portfolio of cementless total knee arthroplasty (TKA) solutions.
“Combined with the Persona TM Femur and the TM Patella, clearance of the Persona TM Tibia allows us to provide a fully cementless total knee solution, furthering our long-standing commitment to enhancing patient experiences and outcomes,” said Dan Williamson, Zimmer Biomet’s Group President, Joint Reconstruction. “We believe this innovative product will position us to re-establish our leadership in the fully cementless primary knee market.”
Zimmer Biomet’s proprietary TM Material is a porous biomaterial made from elemental Tantalum with structural, functional and physiological properties similar to cancellous bone.1-3 With more than 20 years of clinical results, the Company’s TM Material has been used in over two million orthopaedic devices.
“The clearance of the Persona TM Tibia represents a significant step forward for those patients who can benefit from completely cementless total knee replacements that better integrate into the natural bone anatomy for durability, while possibly offering greater patient satisfaction,” said Dr. Moore.
The Persona TM Tibia features new drilling, sizing and insertion instrumentation that are unique to the Persona TM Tibia, while preserving all the anatomic benefits of the Persona Tibia design.4-8 The Persona TM Tibia is another example of how the Persona System continues to redefine personalization by combining Zimmer Biomet’s 20-year porous fixation expertise with the Persona Knee family. As the Company’s most comprehensive knee system, the Persona System offers more anatomically accurate components with finer increments to personalize patient fit and restore the unique identity of every knee.
“The TM tibial tray and pegs fit beautifully to the bone. I believe the instruments for trial prep and implant placement were a clear improvement and an advantage. I also appreciate the time efficiency of using cementless implants,” concluded Dr. Moore.
Zimmer Biomet plans a limited launch of the Persona TM Tibia in the first half of this year, followed by a full commercial launch in the second half of 2018.
About Zimmer Biomet
Founded in 1927 and headquartered in Warsaw, Indiana, Zimmer Biomet is a global leader in musculoskeletal healthcare. We design, manufacture and market orthopaedic reconstructive products; sports medicine, biologics, extremities and trauma products; office based technologies; spine, craniomaxillofacial and thoracic products; dental implants; and related surgical products.
We collaborate with healthcare professionals around the globe to advance the pace of innovation. Our products and solutions help treat patients suffering from disorders of, or injuries to, bones, joints or supporting soft tissues. Together with healthcare professionals, we help millions of people live better lives.
We have operations in more than 25 countries around the world and sell products in more than 100 countries. For more information, visit www.zimmerbiomet.com or follow Zimmer Biomet on Twitter at www.twitter.com/zimmerbiomet.
- Bobyn, et al., Characterization of a New Porous Tantalum Biomaterial for Reconstructive Orthopaedics. 66th Annual AAOS 1999.
- Zhang, et al., Interfacial Frictional Behavior: Cancellous Bone, Cortical Bone, and a Novel Porous Tantalum Biomaterial. Journal of Musculoskeletal Research. 3:4, 245-251, 1999.
- Karageorgiou and Kaplan. Porosity of Biomaterial Scaffolds and Osteogenesis. Biomaterials. 26: 5474-91, 2005.
- Dai, et al. Anatomical Tibial Component Design Can Increase Tibial Coverage and Rotational Alignment Accuracy: A Comparison of Six Contemporary Designs. Knee Surgery Sports Traumatology Arthroscopy 22:2911–2923; KSSTA 2014.
- Indelli, et al. Relationship between Tibial Baseplate Design and Rotational Alignment Landmarks in Primary Total Knee Arthroplasty. Hindawi Publishing Corporation Arthritis. Volume 2015, Article ID 189294.
- Jin, et al. How Much Does the Anatomical Tibial Component Improve the Bony Coverage in Total Knee Arthroplasty. Journal of Arthroplasty. pp. 1-5; 2017.
- Mizu-uchi, et al. Anatomical Shaped Tibial Baseplate Reduced Rotational Alignment Compromise in Total Knee Arthroplasty: Clinical Evaluation with Asian Knees. ORS 2017 Annual Meeting Paper No.0110. 21.Bandi, Marc, et al. Finer Femur and Insert Increments in Total Knee Arthroplasty Facilitate Accurate Balancing and Reduce the Need for Complex Techniques. Abstract number 850; ORS 2014.
- Stulberg and Goyal. Which Tibial Tray Design Achieves Maximum Coverage and Ideal Rotation: Anatomic, Symmetric, or Asymmetric? An MRI-based study. Journal of Arthroplasty. 30(10): 1839, 2015.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the package insert and www.zimmerbiomet.com.
Cautionary Statement Regarding Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning Zimmer Biomet’s expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks, uncertainties and changes in circumstances that could cause actual outcomes and results to differ materially. For a list and description of some of such risks and uncertainties, see Zimmer Biomet’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC). These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in Zimmer Biomet’s filings with the SEC. Forward-looking statements speak only as of the date they are made, and Zimmer Biomet disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Readers of this news release are cautioned not to rely on these forward-looking statements, since there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this news release.
ZB-Corp
CONTACT: Media – Monica Kendrick, 574-372-4989, monica.kendrick@zimmerbiomet.com; Investors – Derek Davis, 574-372-4250, derek.davis@zimmerbiomet.com, or Barbara Goslee, 574-371-9449, barb.goslee@zimmerbiomet.com