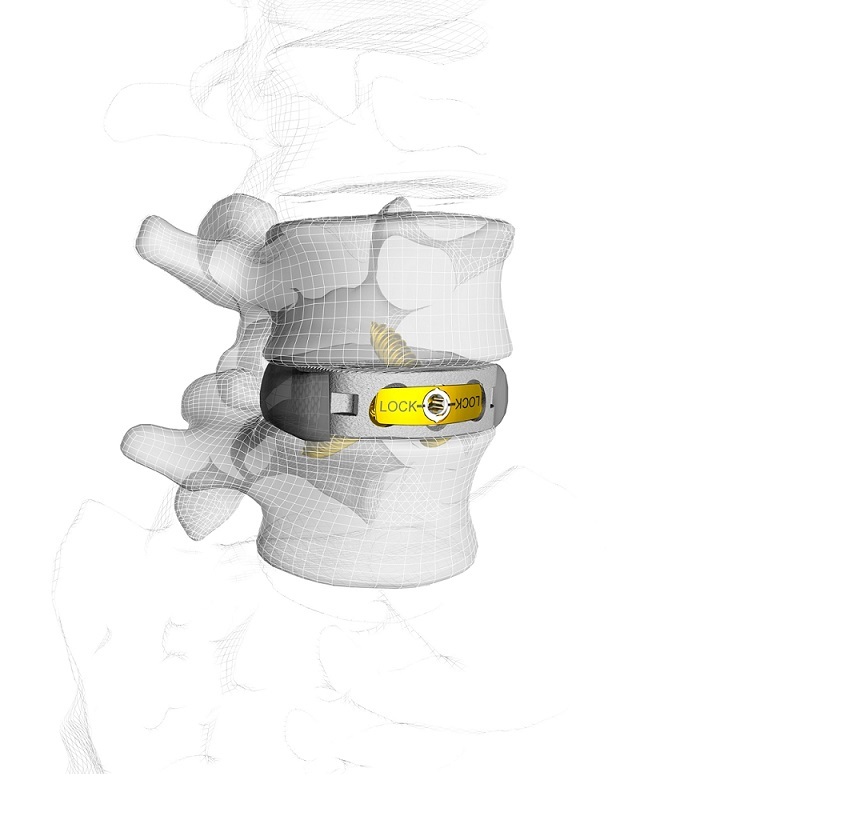
SHELTON, Conn., Sept. 17, 2018 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the commercial launch of the Paramount® Anterior Cervical Cage. The Paramount® Anterior Cervical Cage is a titanium anterior cervical implant with integrated fixation blades. The patented system offers controlled, less invasive blade deployment and maximized bone grafting. The Paramount® Anterior Cervical Cage performed very well during its limited market release and is now fully launched and available, effective immediately. The Paramount® Anterior Cervical Cage is Spine Wave’s second new cervical spine product since 2017 and its seventh major new product launch since 2016.
The Paramount® Anterior Cervical Cage is a titanium implant with a roughened endplate surface and integrated fixation blades. The fixation blades deploy toward and behind the anterior vertebral cortex and away from the spinal cord using a unique threaded deployment mechanism. Used in conjunction with the novel scoring trial, this mechanism gently positions the fixation blades in a controlled manner without force or impaction. This unique design is less invasive because it does not require the exposure necessary to implant non-integrated blades or screws at challenging angles and trajectories. Surgeons using the device can also completely fill the disc space with bone graft, endplate to endplate regardless of endplate anatomy, because of its innovative open face implant design and modular graft cap. The Paramount® Anterior Cervical Cage was co-invented by Sandeep Kunwar, M.D., Medical Director, Bell Neuroscience Institute, Clinical Professor, UCSF Department of Neurological Surgery, San Francisco, California, and Bradley Jones, M.D., Director of the Joint and Spine Center, Dignity Health Medical Center, Redding, California.
“The Paramount® Anterior Cervical Cage is a novel and less invasive solution for anterior cervical interbody fusion,” said Dr. Kunwar. “The unique integrated blade design and in-line implant insertion approach make difficult cases safer and easier in my hands. The procedure can be performed through a smaller insertion window without a struggle to accommodate challenging screw or blade insertion angles.” Dr. Jones continued, “one major goal of our design effort was to eliminate forceful blade impaction from this procedure, and Spine Wave’s engineers did a phenomenal job delivering on that objective. I am very pleased with the finished product.”
The Paramount® Anterior Cervical Cage is the latest example of creative surgeons partnering with our R&D team to develop and deliver a technology that is not only truly differentiated, but also addresses real surgical challenges,” said Spine Wave CEO, Mark LoGuidice. “Prior to 2018, Spine Wave had very little presence in the cervical market segment with the bulk of our revenues being generated by expandable and other MIS technologies designed for the lumbar spine. This launch of the Paramount® system, when combined with the recently launched Proficient® Posterior Cervical Spine System, provides the company with a very strong cervical portfolio and positions us as a full line company to both hospitals and distributors.”
About Spine Wave
Spine Wave is a leader in expandable fusion technologies and is committed to continually delivering highly differentiated products to enable improved and less invasive solutions for spine surgeons and their patients. In addition to the Paramount® Anterior Cervical Cage, Spine Wave also offers many highly differentiated spine technologies, including a full portfolio of expandable devices marketed under the StaXx®, Velocity® and Leva® brand names. These expandable technologies comprise various materials, including PEEK, Titanium (and the combination of the two) that can be utilized in posterior, anterior and lateral surgical approaches. Additionally, Spine Wave has a full suite of less invasive products including our two newest lines, the Proficient® Posterior Cervical Spine System and the GraftMag® Graft Delivery System. The company is expanding rapidly and continues to recruit sales managers and independent distributors to fuel growth. For more information on Spine Wave and its products, please visit www.spinewave.com.
Contact
Terry Brennan, Chief Financial Officer
tbrennan@spinewave.com
(203) 712-1810