August 09, 2017
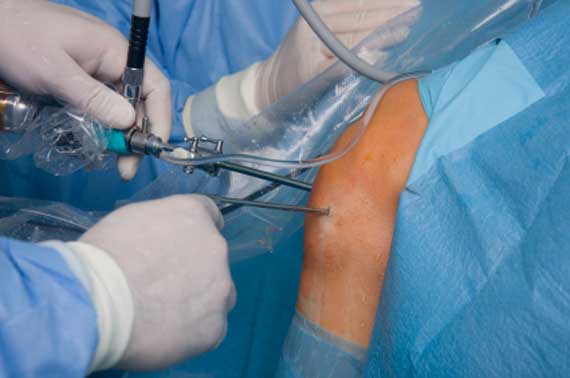
MEMPHIS, Tenn.–(BUSINESS WIRE)–Active Implants, LLC, a company that develops orthopedic implant solutions, today announced enrollment of the 100th patient in clinical trials evaluating the NUsurface® Meniscus Implant for the treatment of persistent knee pain caused by injured or deteriorating meniscus tissue. The surgery was performed by orthopedic surgeon Dr. Wayne Gersoff of Advanced Orthopedics & Sports Medicine Specialists in Denver, Co.
“Treating the 100th NUsurface patient in the U.S. is an important milestone for us as we continue on our mission to fulfill the unmet need in the orthopedic market,” said Ted Davis, president and CEO of Active Implants. “We are pleased that enrollment is going very well and expect to fully enroll all of the patients in the study plan in the coming months. Over the next two to three years, pending FDA clearance, we should be in a position to bring our product to market in the U.S. and fill the gap between minimally invasive meniscus repair and total knee replacement.”
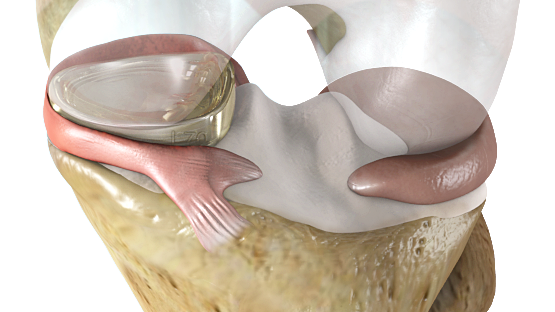
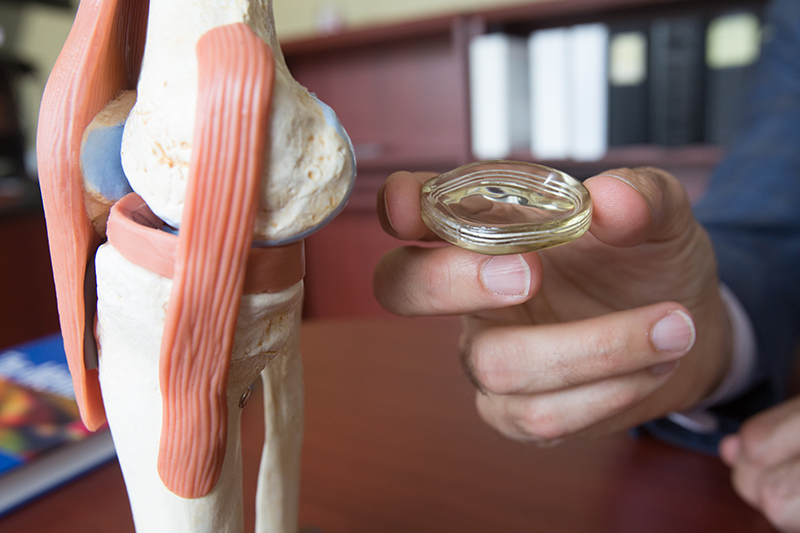
If approved by the Food & Drug Administration, the NUsurface Implant would be the first “artificial meniscus.” The meniscus is a tissue pad between the thigh and shin bones, and once damaged, the meniscus in middle aged patients has a very limited ability to heal itself. Current treatment for a damaged or torn meniscus includes pain management, physical therapy, injections, repair techniques or meniscectomy. It has been estimated that from 700,000 to over 1 million partial meniscectomies are performed annually in the U.S. in an attempt to alleviate pain; however, recent studies have shown that many people who get a meniscectomy continue to experience pain that impacts their quality of life and can eventually lead to knee replacement surgery.
“The NUsurface Implant offers hope for patients who are experiencing persistent knee pain following meniscus injury and surgery, have exhausted non-surgical options and are not yet candidates for total knee replacement,” Dr. Gersoff said. “It’s encouraging to know that with this implant we may be able to alleviate pain and swelling, and delay or even avoid knee replacement surgery – allowing these patients to get back to activities they love.”
The NUsurface Meniscus Implant is made from medical grade polymer and, as a result of its unique materials, composite structure and design, does not require fixation to bone or soft tissues. The NUsurface Implant helps mimic the function of the natural meniscus and redistributes loads transmitted across the knee joint. The NUsurface Meniscus Implant has been used in Europe since 2008 and Israel since 2011.
Active Implants is sponsoring two clinical trials to support regulatory approval from the U.S. Food & Drug Administration (FDA): The VENUS (Verification of the Effectiveness of the NUsurface System) trial is a randomized, controlled study comparing the NUsurface Meniscus Implant compared to non-surgical standard of care and the SUN (Safety Using NUsurface) trial is a single-arm study assessing the safety and effectiveness of the NUsurface Meniscus Implant in restoring function similar to that of a natural, healthy meniscus. The clinical trials are continuing to enroll patients in the following U.S. cities:
VENUS Study
- Albany, New York – Capital Region Orthopaedics (Richard Alfred, MD)
- Boston, Massachusetts – Brigham and Women’s (Andreas Gomoll, MD) and New England Baptist (Brian McKeon, MD)
- Columbus, Ohio – Ohio State University (Christopher Kaeding, MD)
- Durham, North Carolina – Duke University Medical Center (William Garrett, Jr., MD and Claude T. Moorman III, MD)
- Indianapolis, Indiana – OrthoIndy (Jack Farr, MD)
- Memphis, Tennessee – OrthoMemphis (Randall Holcomb, MD)
- New York, New York – Lenox Hill Hospital (Elliott Hershman, MD)
- Richmond, Virginia – Advanced Orthopaedics (Kenneth Zaslav, MD)
- Rochester, New York – University of Rochester Medical (Michael Maloney, MD)
SUN Study
- Arlington, Texas – Baylor Orthopedic and Spine Hospital (Joseph Berman, MD)
- Baton Rouge, Louisiana – Baton Rouge Orthopaedic Clinic (Brent Bankston, MD and Robert Easton, MD)
- Boulder, Colorado – Colorado University Sports Medicine (Eric McCarty, MD)
- Denver, Colorado – Advanced Orthopedics & Sports Medicine Specialists (Wayne Gersoff, MD)
- Long Beach, California – Memorial Orthopaedic Surgical Group (Peter Kurzweil, MD)
- New Orleans, Louisiana – Ochsner Sports Medicine Institute (Deryk Jones, MD)
- Phoenix, Arizona – TOCA, The Orthopedic Clinic Association (Tom Carter, MD)
- Portland, Oregon – Sports Medicine Oregon (Richard Edelson, MD and John Greenleaf, MD)
- Salt Lake City, Utah – Salt Lake Regional Medical Center (Andrew Cooper, MD)
- San Diego, California – Grossmont Orthopaedic Medical Group (Scott Hacker, MD)
- San Francisco, California – St Mary’s Medical Center (William Montgomery, MD)
About Active Implants LLC
Active Implants LLC develops orthopedic implant solutions that complement the natural biomechanics of the musculoskeletal system, allowing patients to maintain or return to an active lifestyle. Active Implants is privately held with headquarters in Memphis, Tennessee. European offices are in Haarlem, The Netherlands, with R&D facilities in Netanya, Israel. For more information, visit www.activeimplants.com.
CAUTION Investigational device. Limited by United States law to investigational use.
Contacts
Merryman Communications
Joni Ramirez, 323-532-0746
joni@merrymancommunications.com