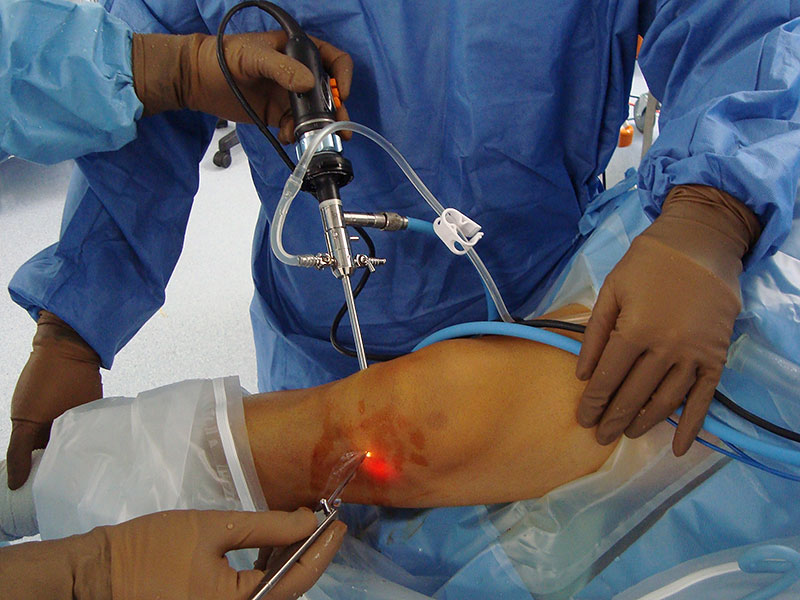
MEMPHIS, Tenn.–(BUSINESS WIRE)–OrthoMemphis and Active Implants, a Memphis-based company that develops orthopedic implant solutions, today announced that the first meniscus replacement procedure in Tennessee was successfully performed.
Dr. Randall Holcomb, sports medicine orthopaedic surgeon and president of OrthoMemphis, was the surgeon selected for the study due to his 30 years of expertise in area of cartilage restoration and transplantation. OrthoMemphis is the only center in Tennessee – and just one of 10 sites nationwide – participating in the VENUS (Verification of the Effectiveness of the NUsurface® System) clinical trial, which is enrolling patients with persistent knee pain after loss of meniscus cartilage to assess the safety and effectiveness of the investigational NUsurface Meniscus Implant compared to non-surgical standard of care.
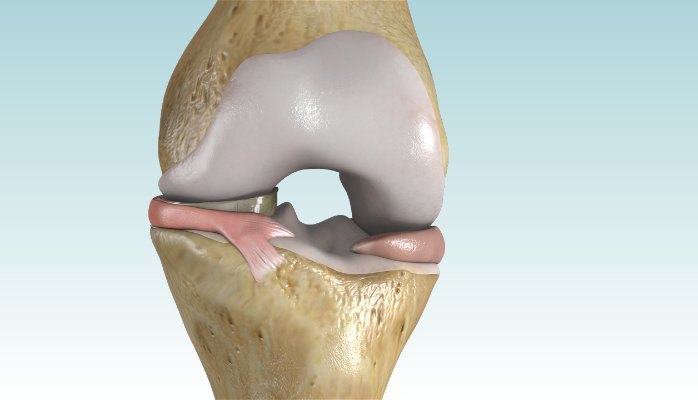
The meniscus is a tissue pad between the thigh and shin bones. Once it is damaged, the meniscus has a very limited ability to heal, and therefore meniscus surgery is performed to remove the torn painful portion of the meniscus. Over 1 million partial meniscectomies are performed in the U.S. every year, more than the total number of hip and knee replacement surgeries combined. While partial meniscectomy surgery is tremendously successful, there is still a subset of patients that continue to experience pain following meniscal surgery. These patients tend to have deteriorating meniscus cartilage, which remains painful and can evolve into arthritis requiring a total knee replacement.
The first patient to receive the NUsurface Meniscus Implant in Tennessee is Memphis resident Dr. Tim Goldsmith. He suffered a knee injury two years ago while practicing twisting lunges during a workout session. This injury led the 60-year-old to undergo alternative treatments to treat the pain, including muscle strengthening physical therapy, injections to relieve pain and finally a partial meniscectomy on his left knee. However, he continued to have consistent knee pain on a daily basis that impacted any activity – from playing golf and interval workout training classes to simply walking up and down stairs.
“There are few options for patients who experience persistent knee pain following meniscus surgery,” Dr. Holcomb said. “We hope the NUsurface implant will act as an artificial meniscus to alleviate their pain and hopefully prevent the onset of degenerative joint disease. Although at this time we cannot make the claim, the NUsurface device may be the solution to slowing down or preventing degenerative arthritis associated with meniscal injuries.”
Dr. Holcomb implanted the device in April 2016 through a small incision in Goldsmith’s knee. Goldsmith was able to quickly return to his work as chief clinical officer at Youth Villages, a private nonprofit dedicated to helping thousands of emotionally and behaviorally troubled children and their families live successfully. Following the full six-week physical therapy regimen with OrthoMemphis’ Steven Chipman,Goldsmith also resumed his favorite pastimes, including golf, spin classes, and interval strength and resistance training. Since his surgery, Goldsmith’s knee has been stable and he has continued his active lifestyle.
“Prior to receiving the NUsurface Meniscus Implant, I felt like I was out of options and had resigned myself to living with pain and waiting for eventual knee replacement,” Goldsmith said. “Now, I am not only rid of the knee pain, but am also experiencing better mobility and flexibility – this gives me hope that I will have the ability to stay as active as I’d like to be for as long as I can.”
The NUsurface Meniscus Implant is inserted into the knee joint through a small incision, and patients typically can go home on the same day of the operation. After surgery, they undergo a six-week rehabilitation program. NUsurface has been used in Europe since 2008 and Israel since 2011.
About the Clinical Trial
As part of the process to gain regulatory approval in the U.S., the VENUS (Verification of the Effectiveness of the NUsurface® System) study will enroll approximately 130 patients at orthopedic centers in the U.S., Europe and Israel. Sites in the U.S. include Indiana (Indianapolis), Massachusetts (Boston), New York (Albany, Rochester and New York), North Carolina (Durham), Ohio (Columbus), Tennessee (Memphis) and Virginia (Richmond). Participants who meet study requirements and agree to enter the trial are randomized to receive either the NUsurface device or non-surgical treatment, which is the current standard of care for patients with persistent knee pain following meniscus surgery. To be eligible for the study, participants must be between the ages of 30 and 75 and have pain after medial meniscus surgery that was performed at least six months ago. To learn more about the VENUS study, please call (844) 680-8951 or visit www.meniscus-trial.com.
About the NUsurface® Meniscus Implant
In the U.S., the NUsurface® Meniscus Implant, from Active Implants LLC, is an investigational treatment for patients with persistent knee pain following medial meniscus surgery. The NUsurface Meniscus Implant is made from medical grade plastic and, as a result of its unique materials, composite structure and design, does not require fixation to bone or soft tissue. The NUsurface device mimics the function of the natural meniscus and redistributes loads transmitted across the knee joint. It is inserted into the knee joint through a small incision, and patients typically can go home soon after the operation. After surgery, patients undergo a six-week rehabilitation program. The NUsurface device has been used clinically in Europe since 2008 and Israel since 2011.
About OrthoMemphis
OrthoMemphis’ team of 18 doctors provides surgical and non-surgical treatment in general orthopedic and subspecialty areas of sports medicine, spine, hand, foot & ankle, hip & knee, total joint replacement, treatments of bone and soft tumors of the extremities (benign and malignant) in children and adults, and workers’ compensation injuries. Fellowship trained surgeons provide expert care in each subspecialty. For more information, go to http://www.orthomemphis.com/.
About Active Implants
Active Implants, LLC develops orthopedic implant solutions that complement the natural biomechanics of the musculoskeletal system, allowing patients to maintain or return to an active lifestyle. Active Implants is privately held with headquarters in Memphis, Tennessee. European offices are in The Netherlands, with R&D facilities in Israel. For more information, visit www.activeimplants.com.
CAUTION Investigational device. Limited by United States law to investigational use.