ATLANTA, July 5, 2017 /PRNewswire/ — MedShape, Inc., the industry leader in orthopaedic devices using advanced functional materials, announced today that it has reached a definitive agreement with CONMED Corporation (Nasdaq:CNMD) for CONMED to acquire the ExoShape® ACL Fixation System. The acquisition, which closed July 3, 2017, includes both the ExoShape FEMORAL and TIBIAL Soft Tissue Fasteners, used to fixate soft tissue grafts in anterior cruciate ligament (ACL) reconstruction surgery.
“The ExoShape System has represented a valuable portion of MedShape’s business since 2011, and this acquisition further demonstrates the success of the development and commercialization efforts invested in the product,” said Kurt Jacobus, MedShape’s CEO. “While we will certainly miss serving our patients, doctors and distributors with this product line, we look forward to focusing our efforts towards growing our core foot and ankle business with new investments.”
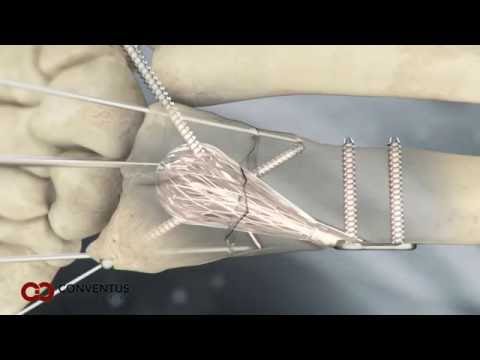
The ExoShape ACL Fixation System represents a breakthrough in soft tissue fixation, as it was the first all-PEEK system to offer a non-rotational deployment and interference fixation approach for ACL reconstruction. Manufactured out of shape memory PEEK Altera®, the ExoShape fasteners are delivered in a low-profile shape for easy insertion and then fully expanded upon instantaneous deployment to effectively compress and secure the soft tissue graft inside the bone tunnel.
About CONMED Corporation
CONMED is a medical technology company that provides surgical devices and equipment for minimally invasive procedures. The Company’s products are used by surgeons and physicians in a variety of specialties, including orthopedics, general surgery, gynecology, neurosurgery and gastroenterology. CONMED has a direct selling presence in 17 countries, and international sales constitute approximately 50% of the Company’s total sales. Headquartered in Utica, New York, the Company employs approximately 3,300 people. For more information, visit www.conmed.com.
About MedShape, Inc.
MedShape, Inc. is a privately held medical device company working to develop and commercialize a portfolio of surgical solutions for foot and ankle and trauma surgeons that use its patented advanced material technologies. For more information, visit: www.medshape.com.
Media Contact:
Jenn Pratt
Carabiner Communications
404.655.2273
jpratt@carabinerpr.com
MedShape, Inc. Contact:
Kathryn Smith, Ph.D.
678.235.3304
Kathryn.smith@medshape.com
CONMED Corporation Contact:
Luke Pomilio
Chief Financial Officer
315.624.3202
LukePomilio@conmed.com
SOURCE MedShape, Inc.