June 06, 2017
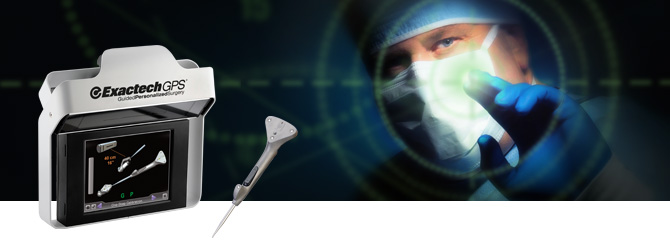
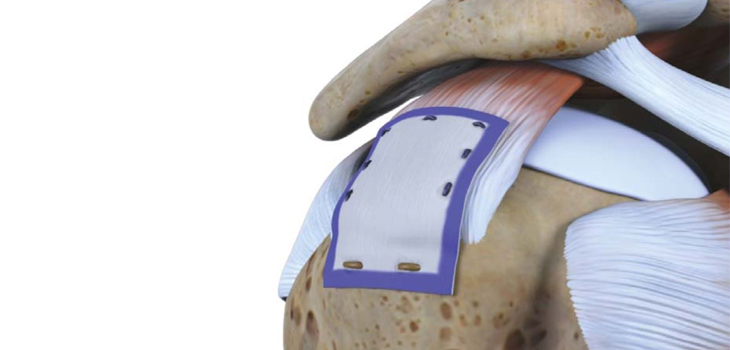
GAINESVILLE, Fla.–(BUSINESS WIRE)–Exactech, Inc. (Nasdaq: EXAC), a developer and producer of bone and joint restoration products and biologic solutions for extremities, knee and hip, announced today a successful first surgery using the new Equinoxe® Preserve stem, a conservative shoulder treatment option designed to preserve humeral bone in shoulder replacement surgery. Additionally, surgeons performed the first U.S. procedures using the ExactechGPS® Shoulder Application.
Orthopaedic surgeon Thomas Wright, MD, performed the first Preserve stem surgery in Gainesville, Fla. “The surgical team and I were pleased with the results of today’s surgery, including the thoughtful implant design, practical instrumentation and relatively simple technique,” Dr. Wright said.
The Preserve stem expands the options offered by the successful Equinoxe®Platform Shoulder System. This new stem is approximately two-thirds the length of the system’s current stem.
“Creating a bone preserving stem that can be used alongside already clinically successful components of our Equinoxe anatomic and reverse implants is an important evolutionary step in our perpetual pursuit of improved clinical outcomes,” said Darin Johnson, Vice President of Marketing, Extremities.
The implant design was developed through the use of CT reconstruction data and clinical studies. Specifically, the implant features a unique polished distal tip to facilitate any need for removal, a straight lateral fin designed for greater rotational stability and plasma coating to aid proximal fixation.
“The technique and implant were created to provide a more bone preserving option within the Equinoxe suite. It was a great pleasure over the last couple of years to collaborate with Drs. Samuel Antuna, Ken Faber, Howard Routman, Joseph Zuckerman and Pierre-Henri Flurin on this innovative improvement. This product demonstrates Exactech’s commitment to working closely with surgeons to improve patient outcomes,” Dr. Wright said.
Dr. Wright and Richard Jones, MD, of Asheville, N.C., also completed the first U.S. surgeries using the ExactechGPS Shoulder Application, a computer-assisted shoulder arthroplasty technology. “This advance in shoulder arthroplasty – being able to execute a preoperative plan in the operating room with real-time visibility into the glenoid vault – has almost limitless possibilities to help patients. Ten years ago, Dr. Sean Grey and I implanted the first Equinoxe reverse shoulder, and that innovation set the pace for the last decade. Now, I believe, ExactechGPS is the breakthrough for the next decade,” Dr. Jones said.
Full U.S. market availability for the Preserve Stem and ExactechGPS Shoulder Application is planned for the beginning of 2018.
About Exactech
Based in Gainesville, Fla., Exactech develops and markets orthopaedic implant devices, related surgical instruments and biologic materials and services to hospitals and physicians. The company manufactures many of its orthopaedic devices at its Gainesville facility. Exactech’s orthopaedic products are used in the restoration of bones and joints that have deteriorated as a result of injury or diseases such as arthritis. Exactech markets its products in the United States, in addition to more than 30 markets in Europe, Latin America, Asia and the Pacific. Additional information about Exactech can be found at http://www.exac.com.
A current investment profile on Exactech (Nasdaq: EXAC) is available online at http://www.hawkassociates.com/profile/exac.cfm. To receive future releases in e-mail alerts, sign up at http://www.hawkassociates.com/about/alert.
This release contains various forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, which represent the company’s expectations or beliefs concerning future events of the company’s financial performance. These forward-looking statements are further qualified by important factors that could cause actual results to differ materially from those in the forward-looking statements. These factors include the effect of competitive pricing, the company’s dependence on the ability of third party manufacturers to produce components on a basis which is cost-effective to the company, market acceptance of the company’s products and the effects of government regulation. Results actually achieved may differ materially from expected results included in these statements.
Contacts
Exactech
Investor contacts:
Jody Phillips, 352-377-1140
Executive Vice President of Finance & Chief Financial Officer
or
Hawk Associates
Julie Marshall or Frank Hawkins, 305-451-1888
EXAC@hawkassociates.com
or
Exactech
Media contact:
Priscilla Bennett, 352-377-1140
Vice President, Corporate & Marketing Communication