April 25, 2017
ALPHARETTA, Ga.–(BUSINESS WIRE)–Cartiva, Inc. (Company), a developer of innovative products for treating cartilage damage and osteoarthritis, announced today that it has completed enrollment and treatment of all 50 patients in a multi-center study evaluating the safety and effectiveness of Cartiva Synthetic Cartilage Implant (SCI) for first carpometacarpal (CMC) joint osteoarthritis at the base of the thumb. The study was conducted at nine sites in Canada and the United Kingdom. Basal thumb arthritis is estimated to afflict more than two million American adults, and up to one-third of post-menopausal women.
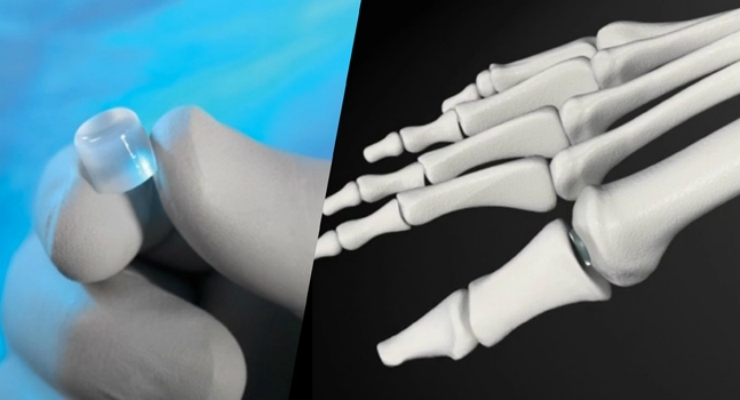
Cartiva SCI is a proprietary biocompatible polymer device designed to mimic natural cartilage. In July 2016, the Company received Premarket Approval from the FDA for use of Cartiva SCI in the treatment of osteoarthritis at the base of the great toe. The thumb implant treats osteoarthritis in patients with diseased or damaged articular surface in the first CMC joint. It is implanted in the metacarpal base to replace damaged cartilage without destroying or removing healthy tissue. The implant’s design minimizes bone resection while preserving the trapezium. This may provide a quicker, less painful recovery than ligament reconstruction tendon interposition (LRTI) surgery or trapeziectomy.
In late February the Company attended a pre-submission meeting with the Food and Drug Administration (FDA) to discuss this new indication. The Company will review the interim six-month results at an investigator meeting next month.
Mr. Philip Sauve, MB BS, FRCSEd (Tr&Ortho) a Consultant Trauma and Orthopaedic Surgeon at Queen Alexandra Hospital in Portsmouth, England who has treated twelve patients with Cartiva, said “So far we have had good results. Their pain is reducing, their grip strength is increasing and so their function is improving. These early results are very promising but we will have to wait to see how the Cartiva implant performs over a longer period of time. For that group of patients who are maybe still working and still very active, I think it’s a really good option.”
“Cartiva SCI for CMC is a unique product that could greatly benefit patients suffering from this debilitating condition,” said Mr. L. Christopher Bainbridge, MB ChB, FRCSEd, Consultant Hand and Peripheral Nerve Surgeon, Pulvertaft Hand Centre, Royal Derby Hospital, and Chief UK Investigator of the study. “With treatment complete, we are now focused on patient follow-up and data analysis.”
“Completion of on-time enrollment was an important milestone for the Company” said Tim Patrick, president and CEO, Cartiva, Inc. “We look forward to working with FDA to make Cartiva SCI for CMC available in the United States for this promising indication.”
Osteoarthritis of the CMC Joint
Osteoarthritis of the CMC joint— also known as thumb basal joint arthritis,—is a debilitating condition impacting 8% to 12% of the general population and as many as 33% of postmenopausal women. It causes pain, swelling, instability, deformity, loss of motion and weakness, making it difficult to perform a variety of tasks, such as turning doorknobs and opening jars. Current surgical options for later-stage patients for fail conservative treatments include joint fusion, total or partial trapeziectomy, arthroplasty, or LRTI (ligament reconstruction and tendon interposition) which has been done for more than 40 years.
About Cartiva, Inc.
Based in Alpharetta, Ga., Cartiva, Inc. develops and markets innovative solutions for patients with cartilage damage and osteoarthritis. Cartiva’s venture investors include New Enterprise Associates and Windham Venture Partners. Additional information is available on the company’s website at www.cartiva.net.
Contacts
Cartiva, Inc.
Peter Pizzo, 770-754-3855
Chief Financial Officer